Abstract
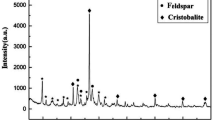
Synthetic magnetic zeolite with Fe3O4 was developed and its adsorption performance of ammonium ions was examined. The influence of pH levels, Fe3O4 content of the magnetic zeolite, magnetization curve of the magnetic zeolite and isotherm curve were examined in batch type experiments. Magnetic zeolite synthesized by a hydrothermal process was observed with less than 12.6 wt% of Fe3O4 content by scanning electron microscope (SEM) and X-ray diffraction (XRD) analysis. With the optimum pH level around 8, qe decreases linearly with the increase of Fe3O4 content. Additionally, the adsorption isotherm of ammonium ions is approximated by a Langmuir equation when the maximum adsorption was obtained at about 1.60 mmolg−1. Such processes as the substitution and thermal desorption methods were successfully introduced to regenerate the magnetic zeolite saturated by ammonium ions, which is shown to be easily separated by magnetic force. Thus, the magnetic zeolite is a potential adsorbent for the removal of ammonium ions with an easy separation method using a magnetic process.
Similar content being viewed by others
References
Bernal MP, Lopez-Real JM (1993) Natural zeolites and sepolite as ammonium and ammonia adsorbent materials. Bioresource Technology 43:27–33, DOI: 10.1016/0960-8524(93)90078-P
Bourlinos AB, Zbori R, Petridis D (2003) A simple route towards magnetically-modified zeolites. Microporous and Mesoporous Materials 58:155–162, DOI: 10.1016/S1387-1811(02)00613-3
Cherneff JB (1973) Magnetic separation of steel cans - Key to solid-waste management. Journal of Milk and Food Technology 36(7):378–382, DOI: 10.4315/0022-2747-36.7.378
Deng Y, Deng C, Qi D, Liu C, Liu J, Zhang X, Zhao D (2009) Synthesis of core/shell colloidal magnetic zeolite microspheres for the immobilization of trypsin. Advanced Material 21(13):1377–1382, DOI: 10.1002/adma.200801766
Eskandarpour A, Onyango MS, Tanahashi M, Ochieng A, Bando Y, Iwai K, Okido M, Asai S (2008) Magnetic fixed-bed column for Cr(VI) removal from aqueous solution using schwertmannite. ISIJ International 48(2):240–244, DOI: 10.2355/isijinternational.48.240
Faghihian H, Moayed M, Firooz A, Iravani M (2014) Evaluation of a new magnetic zeolite composite for removal of Cs+ and Sr2+ from aqueous solutions: Kinetic, equilibrium and thermodynamic studies. Comptes Rendus Chimie 17(2):108–117, DOI: 10.1016/j.crci.2013.02.006
Hartikainen T, Nikkanen JP, Mikkonen R (2005) Magnetic separation of industrial waste waters as an environmental application of superconductivity. IEEE Transactions on Applied Superconductivity 15(2):119–131, DOI: 10.1109/TASC.2005.849660
Huang H, Xiao X, Yan B, Yang L (2010) Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. Journal of Hazardous Materials 175:247–252, DOI: 10.1016/j.jhazmat.2009.09.156
Jung YJ (2019) Removal properties of aqueous ammonium ion with surface modified magnetic zeolite adsorbents. Journal of Wetlands Research 21:152–156 (in Korean)
Lapointe BE, Clark MW (1992) Nutrient inputs from the watershed and coastal eutrophication in the Florida keys. Estuaries 15(4):465–476, DOI: 10.2307/1352391
Laxen DPH, Harrison RM (1977) The highway as a source of water pollution: An appraisal with the heavy metal lead. Water Research 11(1):1–11, DOI: 10.1016/0043-1354(77)90175-0
Liu H, Peng S, Shu L, Chen T, Bao T, Frost RL (2013) Effect of Fe3O4addition on removal of ammonium by zeolite NaA. Journal of Colloid and Interface Science 390:204–210, DOI: 10.1016/j.jcis.2012.09.010
Oliveira LCA, Petkowica DI, Smaniotto A, Pergher SBC (2004) Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Research 38:3699–3704, DOI: 10.1016/j.watres.2004.06.008
Peng X, Luan Z, Zhang H (2006) Montmorillonite-Cu(II)/Fe(III) oxidesmagnetic material as adsorbent for removal of humic acid and its thermal regeneration. Chemosphere 63(2):300–306, DOI: 10.1016/j.chemosphere.2005.07.019
Rozic M, Stefanovic SC, Kurajica S, Vancina V, Hodzic E (2000) Ammonical nitrogen removal from water by treatment with clays and zeolites. Water Research 34(14):3675–3681, DOI: 10.1016/S0043-1354(00)00113-5
Russell JD, Birnie A, Fraser AR (1984) High-gradient magnetic separation (HGMS) in soil clay mineral studies. Clay Minerals 19(5):771–778, DOI: 10.1180/claymin.1984.019.5.07
Towlera PH, Smith JD, Dixonb DR (1996) Magnetic recovery of radium,lead and polonium from seawater samples after preconcentration on a magnetic adsorbent of manganese dioxide coated magnetite. Analytica Chimica Acta 328(1):53–59, DOI: 10.1016/0003-2670(96)00080-3
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal 156:11–24, DOI: 10.1016/j.cej.2009.10.029
Zhang G, Qu J, Liua H, Cooper AT, Wu R (2007) orange II and catalytic regeneration. Chemosphere 68(6):1058–1066, DOI: 10.1016/j.chemosphere.2007.01.081
Zhang T, Zhang X, Yan X, Kong L, Zhang G, Liu H, Qiu J, Yeung KL (2013) Synthesis of Fe3O4@ZIF-8 magnetic core-shell microspheres and their potential application in a capillary microreactor. Chemical Engineering Journal 228:398–404, DOI: 10.1016/j.cej.2013.05.020
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamimoto, Y., Hagio, T., Jung, YJ. et al. Development of Synthetic Magnetic Zeolite Adsorbents and Application to Ammonium Ion Removal. KSCE J Civ Eng 24, 1395–1399 (2020). https://doi.org/10.1007/s12205-020-2185-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12205-020-2185-5