Abstract
Milled olive stones are evaluated as a biosorbent for the removal of heavy metals such as Cd (II), Cu (II), Pb(II) and Cr(VI) from aqueous effluents. To this end, thermodynamic and kinetic studies for single and multimetal systems are performed through batch equilibrium isotherms. The biosorbent was characterized by elemental and FTIR analysis and scanning electron microscopy. The effect of the different parameters, such as contact time, pH, amount of adsorbent and initial metal concentration, on the sorption process is also investigated. The maximum removal percentage for 1 mg L−1 of cadmium, copper, and lead was 77.4%, 80.5%, and 94.5%, respectively, at pH 6 with 5 g L−1 of sorbent. In the case of Cr(VI), a removal percentage of 46% was obtained in 2 h at pH 2 using a larger amount of sorbent (10 g L−1) and an initial concentration of 2 mg L−1. Equilibrium data were analyzed by applying different adsorption isotherm models, resulting in—a good agreement with—the Langmuir model with maximum capacities of 0.557, 0.3 and 0.581 mg g−1 for Cu(II), Cd(II) and Pb(II), respectively, whereas for Cr(VI), the Temkin model provided the best fit with a maximum capacity of 2.34 mg g−1. The kinetic data fitted well into the pseudo-second-order model which allowed the adsorption rate constants to be calculated. Cd(II) resulted to have the highest kinetic constant, followed by Cu(II), Cr(VI) and Pb(II). The results showed that milled olive stones can be used as a biosorbent for the removal of these metals from aqueous solutions.
Article Highlights
-
Trace concentrations of Cu(II), Cd(II), Pb(II), and Cr(VI) were adsorbed by olive stones.
-
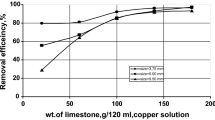
Removals of 77.4%, 80.5 and 94.5% were obtained for Cu(II), Cd(II), or Pb(II) at pH 6.
-
For Cr(VI), a removal percentage of 46% was obtained at pH 2 with 10 g L−1 of sorbent.
-
Langmuir isotherm model was fitted to Cu(II), Cd(II), and Pb(II) adsorption isotherm data.
-
Cd(II) has the highest pseudo-second-order kinetic constant, followed by Cu(II), Cr(VI) and Pb(II).
-
Milled olive stone is an effective and cheaper biosorbent to remove toxic metals.








Similar content being viewed by others
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Tech 3(4):74–102
Abdel Salam OE, Reiad NA, ElShafei MM (2011) A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J Adv Res 2:297–303. https://doi.org/10.1016/j.jare.2011.01.008
Abudaia JA, Sulyman MO, Elazaby KY, Ben-Ali SM (2013) Adsorption of Pb(II) and Cu (II) from aqueous solution onto activated carbon prepared from dates stones. Int J Environ Sci Dev 4:191–195. https://doi.org/10.7763/IJESD.2013.V4.333
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2014) Preparation of activated carbon from olive stone waste: optimization study on the removal of Cu2+, Cd2+, Ni2+, Pb2+, Fe2+, and Zn2+ from aqueous solution using response surface methodology. J Dispers Sci Technol 35:913–925. https://doi.org/10.1080/01932691.2013.809506
Blázquez G, Hernáinz F, Calero M, Ruiz-Núñez LF (2005) Removal of cadmium ions with olive stones: the effect of some parameters. Proc Biol 40:2649–2654. https://doi.org/10.1016/j.procbio.2004.11.007
Blázquez G, Hernáinz F, Calero M, Martin-Lara MA, Tenorio G (2009) The effect of pH on the biosorption of Cr(III) and Cr(VI) with olive stone. Chem Eng J 148:473–479. https://doi.org/10.1016/j.cej.2008.09.026
Blázquez G, Martín-Lara MA, Dionisio-Ruiz E, Tenorio G, Calero M (2011) Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. J Ind Eng Chem 17:824–833. https://doi.org/10.1016/j.jiec.2011.08.003
Bohli T, Ouederni A, Fiol N, Villaescusa I (2012) Uptake of Cd2+ and Ni2+ metal ions from aqueous solutions by activated carbons derived from waste olive stones. Int J Chem Eng Appl 3:232–236. https://doi.org/10.7763/IJCEA.2012.V3.192
Budinova T, Petrov N, Razvigorova M, Parra J, Galiatsatou P (2006) Removal of arsenic(III) from aqueous solution by activated carbons prepared from solvent extracted olive pulp and olive stones. Ind Eng Chem Res 45:1896–1901. https://doi.org/10.1021/ie051217a
Chand P, Pakade YB (2013) Removal of Pb from water by adsorption on apple pomace: equilibrium, kinetics, and thermodynamics studies. J Chem (Hindawi, Online) Article ID 164575. https://doi.org/10.1155/2013/164575
Deliyanni EA, Kyzas GZ, Triantafyllidis KS, Matis KA (2015) Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem 13:699–708
El-Sayed GO, Dessouki HA, Ibrahiem SS (2011) Removal of Zn (II), Cd (II) and Mn(II) from aqueous solutions by adsorption on maize stalks. MJAS 15:8–21
Etorki AM, El-Rais M, Mahabbis MT, Moussa NM (2014) Removal of some heavy metals from wastewater by using of fava beans. Am J Anal Chem 05:225. https://doi.org/10.4236/ajac.2014.54028
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Gao H, Liu Y, Zeng G, Xu W, Li T, Xia W (2008) Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste—rice straw. J Hazard Mater 150:446–452. https://doi.org/10.1016/j.jhazmat.2007.04.126
Ghaneiam MT, Bhatnagar A, Ehrampoush MH, Amrollahi M, Jamsihi B, Dehvari M, Taghavi M (2017) Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: kinetic modeling studies. Int J Environ Sci Technol 14:331–340. https://doi.org/10.1007/s13762-016-1216-8
Ghrab S, Benzina M, Lambert SD (2017) Copper adsorption from wastewater using bone charcoal. Adv Mat Phys Chem 07:139. https://doi.org/10.4236/ampc.2017.75012
Hernáinz F, Calero M, Blázquez G, Martin-Lara MA, Tenorio G (2008) Comparative study of the biosorption of cadmium(II), chromium(III), and lead(II) by olive stone. Environ Prog 27:469–478. https://doi.org/10.1002/ep.10299
Johnson TA, Jain N, Joshi HC, Prasad S (2008) Agricultural and agro-processing wastes as low cost adsorbents for metal removal from wastewater: a review. J Sci Ind Res (India) 67:647–658
Kyzas GZ (2012) Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 5(10):1826–1840. https://doi.org/10.3390/ma5101826
Martín-Lara MA, Blázquez G, Trujillo MC, Pérez A, Calero M (2014) New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J Clean Prod 81:120–129. https://doi.org/10.1016/j.jclepro.2014.06.036
Martín-Lara MA, Calero de Hoces M, Ronda Gálvez A, Pérez Muñoz A, Trujillo Miranda MC (2016) Assessment of the removal mechanism of hexavalent chromium from aqueous solutions by olive stone. Water Sci Technol 73:2680–2688. https://doi.org/10.2166/wst.2016.081
Mishra A, Dubey A, Shinghal S (2015) Biosorption of chromium(VI) from aqueous solutions using waste plant biomass. Int J Environ Sci Technol 12:1415–1426. https://doi.org/10.1007/s13762-014-0516-0
Nakano Y, Takeshita K, Tsutsumi T (2001) Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res 35:496–500. https://doi.org/10.1016/S0043-1354(00)00279-7
Okafor PC, Okon P, Daniel E, Ebenso E (2012) Adsorption capacity of coconut (Cocos nucifera L.) shell for lead, copper, cadmium and arsenic from aqueous solutions. Int J Electrochem Sci 7:12354–12369. http://www.electrochemsci.org/papers/vol7/71212354.pdf
Pintor AMA, Ferreira CIA, Pereira JC, Correia P, Silva SP, Vilar VP, Botelho CM, Boaventura RA (2012) Use of cork powder and granules for the adsorption of pollutants: a review. Water Res 46:3152–3166. https://doi.org/10.1016/j.watres.2012.03.048
Ronda A, Martín-Lara MÁ, Blázquez G, Moreno N, Calero M (2014) Copper biosorption in the presence of lead onto olive stone and pine bark in batch and continuous systems. Environ Prog Sustain Energy 33:192–204. https://doi.org/10.1002/ep.11780
Saka C, Sahin O, Küçük MM (2012) Applications on agricultural and forest waste adsorbents for the removal of lead (II) from contaminated waters. Int J Environ Sci Technol 9:379–394. https://doi.org/10.1007/s13762-012-0041-y
Sanchooli Moghaddam M, Rahdar S, Taghavi M (2016) Cadmium removal from aqueous solutions using saxaul tree ash. Iran J Chem Chem Eng 35:45–52
Sarin V, Pant KK (2006) Removal of chromium from industrial waste by using eucalyptus bark. Bioresour Technol 97:15–20. https://doi.org/10.1016/j.biortech.2005.02.010
Şen A, Pereira H, Olivella MA, Villaescusa I (2015) Heavy metals removal in aqueous environments using bark as a biosorbent. Int J Environ Sci Tech 12:391–404. https://doi.org/10.1007/s13762-014-0525-z
Sfaksi Z, Azzouz N, Abdelwahab A (2014) Removal of Cr(VI) from water by cork waste. Arab J Chem 7:37–42. https://doi.org/10.1016/j.arabjc.2013.05.031
Vinodhini V, Das N (2010) Relevant approach to assess the performance of sawdust as adsorbent of chromium (VI) ions from aqueous solutions. Int J Environ Sci Technol 7(1):85–92. https://doi.org/10.1007/bf03326120
Acknowledgements
M. Ben Amar acknowledges the financial support of the Faculty of Science (University of Sfax, Tunisia) and of The Environmental and Analytical Chemistry Research Group of the Department of Chemistry (University of Girona, Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amar, M.B., Walha, K. & Salvadó, V. Evaluation of Olive Stones for Cd(II), Cu(II), Pb(II) and Cr(VI) Biosorption from Aqueous Solution: Equilibrium and Kinetics. Int J Environ Res 14, 193–204 (2020). https://doi.org/10.1007/s41742-020-00246-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00246-5