Abstract
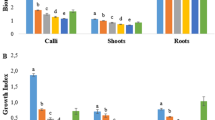
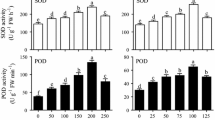
Calycosin-7-O-β-D-glucoside (CG), a methoxylated isoflavonoid in Astragalus membranaceus Fisch. (Bunge), has a wide range of biological activities. Adventitious root culture is an alternative source to obtain active compounds. To improve CG production in A. membranaceus adventitious roots (AMARs), effect of nitrogen concentration, and phosphate concentration on accumulation of biomass, CG, and hydrogen peroxide were investigated. A low concentration of nitrogen promoted growth and CG accumulation in AMARs. Maximum biomass and CG productivity were obtained at a nitrogen concentration of 3 mM. Nitrate, rather than ammonium, was suitable for CG production. Phosphate concentration did not influence biomass accumulation; however, high concentration of phosphate (2.5 mM) was most favorable for CG accumulation. Furthermore, moderate levels of hydrogen peroxide promoted CG accumulation. Optimal concentrations of nitrogen and phosphate were used in scale-up culture of AMARs using a bioreactor. There was a 8- and 2- fold increase in the CG content and antioxidant activity, respectively, using a B5 medium containing 3 mM NO3− and 2.5 mM phosphate when compared to the standard B5 medium, which was equivalent to those observed in field-grown plants. Therefore, optimization of nitrogen and phosphate concentration in the medium is a simple and effective method to economically improve production of CG in AMARs.






Similar content being viewed by others
References
Awah FM, Uzoegwu PN, Ifeonu P, Oyugi JO, Rutherford J, Yao XJ, Fehrmann F, Fowke KR, Eze MO (2012) Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem 131:1279–1286
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59:411–416
Černý M, Habánová H, Berka M, Luklová M, Brzobohatý B (2018) Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int J Mol Sci 19:2812
Chinese Pharmacopoeia Commission (2015) Huangqi. In: Pharmacopoeia of the People’s republic of China. China Medical Science Press, Beijing, pp 302–303
Cui XH, Murthy HN, Wu CH, Paek KY (2010) Adventitious root suspension cultures of Hypericum perforatum: effect of nitrogen source on production of biomass and secondary metabolites. In Vitro Cell Dev Biol-Plant 46:437–444
Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, Zhang H, Yang S (2014) Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res 28:1275–1283
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168:17–20
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LS (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420
Huang T, Gao WY, Wang J, Cao Y, Zhao YX, Huang LQ, Liu CX (2010) Selection and optimization of a high-producing tissue culture of Panax ginseng C. A. Meyer. Acta Physiol Plant 32:765–772
Kim JH, Chang EJ, Oh HI (2005) Saponin production in submerged adventitious root culture of Panax ginseng as affected by culture conditions and elicitors. Asia Pac J Chem Eng 13:87–91
Koricheva J, Larsson S, Haukioja E, Keinanen M (1998) Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83:212–226
Lee EJ, Paek KY (2012) Effect of nitrogen source on biomass and bioactive compound production in submerged cultures of Eleutherococcus koreanum Nakai adventitious roots. Biotechnol Prog 28:508–514
Lee Y, Lee DE, Lee HS, Kim SK, Lee WS, Kim SH, Kim MW (2011) Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morus alba L.). Plant Cell Tiss Org Cult 105:9–19
Liu J, Chen HB, Guo BL, Zhao ZZ, Liang ZT, Yi T (2011) Study of the relationship between genetics and geography in determining the quality of Astragali Radix. Biol Pharm Bull 34:1404–1412
Liu J, Lan X, Lv S, Bao R, Yuan Y, Wu S, Quan X (2019) Salicylic acid involved in chilling-induced accumulation of calycosin-7-O-β-D-glucoside in Astragalus membranaceus adventitious roots. Acta Physiol Plant 41:120
Liu P, Zhao H, Luo Y (2017) Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 8:868–886
Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW (2002) Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem 50:4861–4866
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Dandin VS, Paek KY (2014) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15:1–17
Murthy HN, Praveen N (2012) Influence of macro elements and nitrogen source on adventitious root growth and withanolide-a production in Withania somnifera (L.) Dunal. Nat Prod Res 226:466–473
Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Medina-Bolivar F (2014) Differential induction of antioxidant stilbenoids in hairy roots of Vitis rotundifolia treated with methyl jasmonate and hydrogen peroxide. Plant Physiol Biochem 74:50–69
Pan H, Li X, Cheng X, Wang X, Fang C, Zhou T, Chen J (2015) Evidence of calycosin-7-O-β-D-glucoside's role as a major antioxidant molecule of Astragalus membranaceus Bge. Var. mongholicus (Bge.) Hsiao plants under freezing stress. Environ Exp Bot 109:1–11
Park YJ, Thwe AA, Li X, Kim YJ, Kim JK, Arasu MV, Al-Dhabi NA, Park SU (2015) Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J Agric Food Chem 63:8862–8869
Rajesh M, Sivanandhan G, Arun M, Vasudevan V, Theboral J, Girija S, Manickavasagam M, Selvaraj N, Ganapathi A (2014) Factors influencing podophyllotoxin production in adventitious root culture of Podophyllum hexandrum Royle[J]. Acta Physiol Plant 36:1009–1021
Saxena I, Srikanth S, Chen Z (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Sci 7:570
Sivakumar BG, Yu KW, Paek KY (2005) Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng Life Sci 5:333–342
Tian X, Chen S, Zhang Y, Chen L, Guo X, Xu Z, Liu H, Hu P, Chen Z, Li Z, Huang C (2018) Absorption, liver first-pass effect, pharmacokinetics and tissue distribution of calycosin-7-O-β-D-glucoside (C7G) and its major active metabolite, calycosin, following oral administration of C7G in rats by LC-MS/MS. J Pharm Biomed Anal 148:350–354
Tsai CC, Wu HH, Chang CP, Lin CH, Yang HH (2019) Calycosin-7-O-β-D-glucoside reduces myocardial injury in heat stroke rats. J Formos Med Assoc 118:730–738
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193–199
Wu SQ, Lian ML, Gao R, Park SY, Piao XC (2011) Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell Dev Biol-Plant 47:719–724
Yin S, Gao W, Liang Y, Wang J, Liu H, Wei C, Zuo B (2013) Influence of sucrose concentration and phosphate source on biomass and metabolite accumulation in adventitious roots of Pseudostellaria heterophylla. Acta Physiol Plant 35:1579–1585
Yin S, Zhang Y, Gao W, Wang J, Man S, Liu H (2014) Effects of nitrogen source and phosphate concentration on biomass and metabolites accumulation in adventitious root culture of Glycyrrhiza uralensis Fisch. Acta Physiol Plant 36:915–921
Yu KW, Gao WY, Hahn EJ, Paek KY (2001) Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in ginseng (Panax ginseng C. A. Meyer). J Plant Biol 44:179–184
Zhang J, Xue X, Yang Y, Ma W, Han Y, Qin X (2018) Multiple biological defects caused by calycosin-7-O-β-D-glucoside in the nematode Caenorhabditis elegans are associated with the activation of oxidative damage. J Appl Toxicol 38:801–809
Zhao C, Liu Q (2008) Growth and physiological responses of Picea asperata seedlings to elevated temperature and to nitrogen fertilization. Acta Physiol Plant 31:163–173
Zhu JJ, Li YR, Liao JX (2013) Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. leaves. Plant Physiol Biochem 73:427–433
Acknowledgments
This study was funded by the National Natural Science Foundation of China (21462044 and 30860036) and the Natural Science Foundation of Jilin Province of China (201115227).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Ming Cheng
Rights and permissions
About this article
Cite this article
Jin, H., Yu, Y., Hu, S. et al. Effect of nitrogen concentration, source, and phosphate concentration on accumulation of biomass and calycosin-7-O-β-D-glucoside in Astragalus membranaceus adventitious roots. In Vitro Cell.Dev.Biol.-Plant 56, 407–414 (2020). https://doi.org/10.1007/s11627-020-10072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10072-1