Abstract
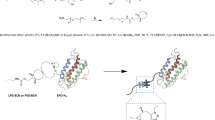
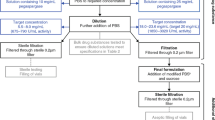
Erythropoietin (EPO) is a glycoprotein that acts as a cytokine for erythrocyte precursors in the bone marrow, thus, being regulating erythropoiesis. Since polypeptides such as EPO are less stable, a breakthrough for protein drugs is needed. To avoid these limitations, a long-acting conjugate was developed in this study, where we aimed to formulate a long-acting EPO conjugate consists of EPO linked to the immunoglobulin Fc fragment. Here we performed RP-HPLC and SE-HPLC analyses and biological activity assays after buffer exchange under various conditions for the development of liquid stabilizer formulations of a long-acting EPO conjugate and to find conditions that its maintain purity and activity. First, the ability of various stabilizers to stabilize the long-acting EPO conjugate was analyzed, and it was confirmed that a specific stabilizer (polysorbate, mannitol, and sodium chloride)maintained the long-acting EPO conjugate. In addition, following long-term storage stability, it was confirmed that the liquid formulation kept the long-acting EPO conjugate stable at 4ºC for 12 months. Even without neutral amino acids, the liquid formulation from this study can provide excellent storage stability for EPO, making it more economically beneficial than other stabilizers and lyophilizing agents.
Similar content being viewed by others
References
Mijake, T., C. K. Kung, and E. Goldwasser (1997) Purification of human erythropoietin. J. Biol. Chem. 25: 5558–5564.
Eschbach, J. W., J. C. Egrie, M. R. Downing, J. K. Browne, and J. W. Adamson (1987) Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I. and II. clinical trial. N Engl. J. Med. 316: 73–78.
Krantz, S. B. (1991) Erythropoietin. Blood. 77: 419–434.
Shoemaker, C. B. and L. D. Mitsock (1986) Murine erythropoietin gene: cloning, expression, and human gene homology. Mol. Cell Biol. 6: 849–858.
Solaro, R., F. Chieliini, and A. Battisti (2010) Targeted delivery of protein drugs by nanocarriers. Materials. 3: 1928–1980.
Raza, F., H. Zafar, Y. Zhu, Y. Ren, A. Ullah, A. U. Khan, X. He, H. Han, M. Aquib, K. O. Boakye-Yiadom, and L. Ge (2018) A. review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics. 10: El 6.
Sada, E., K. Katoh, K. Yamanaka, and Y. Itakura (1991) Resistance to proteolysis of antibody ligands modified with polyethylene glycol. J. Ferment. Bioeng. 71: 137–139.
Lim, S. I., M. H. Jang, D. J. Kim, S. M. Bae, and S. C. Kwon (2011) Cobalt(III)-induced hexamerization of PEGylated insulin. Int. J. Biol. Macromol. 49: 832–837.
Francesco, M. V. (2001) Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 22: 405–417.
Salfeld, I., H. G. Göttlinger, R. A. Sia, R. E. Park, J. G. Sodroski, and W. A. Haseltine (1990) A. tripartite HIV-1 tat-env-rev fusion protein. EMBO J. 9: 965–970.
Yeh, P., D. Landais, M. Lemaître, I. Maury, J. Y. Crenne, J. Becquart, A. Murry-Brelier, F. Boucher, G. Montay, R. Fleer, P. H. Hirel, J. F. Mayaux, and D. Klatzmann (1992) Design of yeast-secreted albumin derivatives for human therapy: biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proc. Natl. Acad. Sci. USA. 89: 1904–1908.
Lu, H., P. Yeh, J. D. Guitton, C. Mabilat, F. Desanlis, I. Maury, Y. Legrand, J. Soria, and C. Soria (1994) Blockage of the urokinase receptor on the cell surface: construction and characterization of a hybrid protein consisting of the N-terminal fragment of human urokinase and human albumin. FEBS Lett. 356: 56–59.
Geren, L. M., J. Stonehuerner, D. J. Davis, and F. Millett (1983) The use of a water-soluble carbodiimide to cross-link cytochrome c to plastocyanin. Biochim. Biophys. Acta. 724: 62–68.
Slusarewicz, P., K. Zhu, and T. Hedman (2010) Kinetic characterization and comparison of various protein crosslinking reagents for matrix modification. J. Mater. Sci. Mater. Med. 21: 1175–1181.
Wang, W. (1999) Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 185: 129–188.
Norde, W. (1986) Adsorption of protein from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 25: 267–340.
Duncan, M. R., J. M. Lee, and M. P. Warchol (1995) Influence of surfactants upon protein / peptide adsorption to glass and polypropylene. Int. J. Pharm. 120: 179–188.
Remmele, R. L., S. Krishnan, and W. J. Callahan (2012) Development of stable lyophilized protein drug products. Curr Pharm. Biotechnol. 13: 471–496.
Wang, W. (2000) Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 203: 1–60.
Tarelli, E., A. Mire-Sluis, H. A. Tivnann, B. Bolgiano, D. T. Crane, C. Gee, X. Lemercinier, M. L. Athayde, N. Sutcliffe, P. H. Corran, and B. Rafferty (1998) Recombinant human albumin as a stabilizer for biological materials and for the preparation of international reference reagents. Biologicals. 26: 331–346.
Ucan-Marin, F., A. Arukwe, A. S. Mortensen, G. W. Gabrielsen, and R. J. Letcher (2010) Recombinant albumin and transthyretin transport proteins from two gull species and human: chlorinated and brominated contaminant binding and thyroid hormones. Environ. Sci. Technol. 44: 497–504.
Zhu, I., J. W. Wooh, J. J. Hou, B. S. Hughes, P. P. Gray, and T. P. Munro (2012) Recombinant human albumin supports single cell cloning of CHO. cells in chemically defined media. Biotechnol Prog. 28: 887–891.
Cho, S. H., H. S. Lim, J. L. Ghim, S. Choe, U. J. Kim, J. A. Jung, and K. S. Bae (2009) Pharmacokinetic, tolerability, and bioequivalence comparison of three different intravenous formulations of recombinant human erythropoietin in healthy Korean adult male volunteers: An open-label, randomized-sequence, three-treatment, three-way crossover study. Clin Ther. 31: 1046–1053.
Kwon, S. C., G. S. Lee, J. Y. Han, M. Lee, Y. M. Lee, W. S. Koh, and J. Y. Kim (2010) Genotoxicity studies on HM10760A, recombinant human erythropoietin conjugated to globin fragment. Drug Chem. Toxicol. 33: 152–159.
Acknowledgements
The authors declare no conflict of interest.
Neither ethical approval nor informed zonsent was required for this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, HK., Hong, S.H., Bae, S.M. et al. A Liquid Formulation of a Long-acting Erythropoietin Conjugate. Biotechnol Bioproc E 25, 117–125 (2020). https://doi.org/10.1007/s12257-019-0194-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0194-3