Abstract
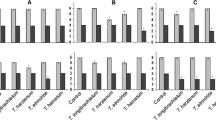
Thirty four Trichoderma isolates were systematically screened for induction of systemic resistance in grapevines against powdery mildew disease. Trichoderma 5R, NAIMCC-F-01769, NAIMCC-F-01812 and NAIMCC-F-01951 were selected for field trials based on initial screening on potted plants. All the four isolates tested positive for production of plant growth promoting bio-chemicals, indole acetic acid, siderophore, ammonia, hydrogen cyanide and phosphate solubilization and produced chitinase, β-1,3-glucanase, cellulase, amylase and protease. These four isolates were compatible with each other and were tested singly or as mixtures. In the three grapevine field trials conducted during the fruiting growth phase 2015–16 and 2016–17 and the vegetative growth phase 2016, the maximum reduction in powdery mildew severity was found in vines treated with 5R + NAIMCC-F-01812, followed by vines treated with 5R alone showing that the efficacy of 5R was slightly enhanced when used along with NAIMCC-F-01812. Total phenol contents, and chitinase and β-1, 3-glucanase activities were highest in leaves of vines treated with 5R + F-01812 corroborating the field observations. The peroxidase and polyphenol oxidase activities were also high in leaves of vines under this treatment. Multilocus analysis using partial nucleotide sequences of act, rpb2 and tef1 genes showed phylogenetic affiliations of all four isolates to T. asperelloides.


Similar content being viewed by others
References
Altomare C, Norvell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol 65:2926–2933
Aziz A, Verhagen B, Magnin-Robert M, Couderchet M, Clément C, Jeandet P, Trotel-Aziz P (2016) Effectiveness of beneficial bacteria to promote systemic resistance of grapevine to gray mold as related to phytoalexin production in vineyards. Plant Soil 405:141–153
Bennett FG, Westcott B (1982) Field assessment of resistance to powdery mildew in mature wheat plants. Plant Pathol 31:261–268
Brotman Y, Lisec J, Meret M, Chet I, Willmitzer L, Viterbo A (2012) Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiol 158:139–146
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Cutler HG, Himmelsbach DS, Arrendale RF, Cox RH (1989) Koninginin a: a novel plant growth regulator from Trichoderma koningii. Agric Biol Chem 53:2605–2611
Cutler HG, Jacyno JM, Phillips RS, Vontersch RL, Cole PD, Montemurro N (1991) Cyclonerodiol from a novel source, Trichoderma koningii: plant growth regulatory activity. Agric Biol Chem 55:243–244
Darlu P, Lecointre G (2002) When does the incongruence length difference test fail? Mol Biol Evol 19:432–437
Dixit R, Singh RB, Singh HB (2015) Screening of antagonistic potential and plant growth promotion activities of Trichoderma spp. and fluorescent Pseudomonas spp. isolates against Sclerotinia sclerotiorum causing stem rot of French bean. Legume Res 38:375–381
Elad Y (2000) Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot 19:709–714
Fuchs JG, Moënne-Loccoz Y, Défago G (1997) Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Dis 81:492–496
Gupta P, Ravi I, Sharma V (2013) Induction of β-1, 3-glucanase and chitinase activity in the defense response of Eruca sativa plants against the fungal pathogen Alternaria brassicicola. J Plant Interact 8:155–161
Hanson LE, Howell CR (2004) Elicitors of plant defense responses from biocontrol strains of Trichoderma virens. Phytopathol 94:171–176
Horsfall JG, Heuberger JW (1942) Measuring magnitude of a defoliation disease in tomatoes. Phytopathol 32:226–232
Howell CR, Hanson LE, Stipanovic RD, Puckhaber LS (2000) Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathol 90:248–252
Hoyos-Carvajal L, Orduz S, Bissett J (2009) Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control 51:409–416
Jangid PK, Mathur PN, Mathur K (2004) Role of chitinase and β-1,3 glucanase elicitation in the Trichoderma harzianum induced systemic resistance in capsicum. Indian Phytopathol 57:217–221
Jetiyanon K, Kloepper JW (2002) Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control 24:285–291
Jogaiah S, Abdelrahman A, Tran LSP, Shin-ichi I (2013) Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J Exp Bot 64:3829–3842. https://doi.org/10.1093/jxb/ert212
Joosten MH, De-Wit PJ (1989) Identification of several pathogenesis-related proteins in tomato leaves inoculated with Cladosporium fulvum (syn. Fulvia fulva) as 1,3-β-glucanases and chitinases. Plant Physiol 89:945–951
Khan J, Ooka JJ, Miller SA, Madden LV, Hoitink HAJ (2004) Systemic resistance induced by Trichoderma hamatum 382 in cucumber against Phytophthora crown rot and leaf blight. Plant Dis 88:280–286
Koike N, Hyakumachi M, Kageyama K, Tsuyumu S, Doke N (2001) Induction of systemic resistance in cucumber against several diseases by plant growth-promoting fungi: lignification and superoxide generation. Eur J Plant Pathol 107:523–533. https://doi.org/10.1023/A:1011203826805
Konappa N, Krishnamurthy S, Siddaiah CN, Ramachandrappa NS, Chowdappa S (2018) Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt J Biol Pest Con 28:63
Legler SE, Pintye A, Caffi T, Gulyas S, Bohár G, Rossi V, Kiss L (2016) Sporulation rate in culture and mycoparasitic activity, but not mycohost specificity, are the key factors for selecting Ampelomyces strains for biocontrol of grapevine powdery mildew (Erysiphe necator). Eur J Plant Pathol 144:723–736
Li Y, Gu Y, Li J, Xu M, Wei Q, Wang Y (2015) Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front Microbiol 6:883. https://doi.org/10.3389/fmicb.2015.00883
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16:1799–1808
Lowry OH, Rosebrough NJ, Fair AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marques APGC, Pires C, Moreira H, Rangel AOSS, Castro PML (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42:1229–1235
Meyer GD, Bigirimana J, Elad Y, Hofte M (1998) Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur J Plant Pathol 104:279–286
Perazzolli M, Dagostin S, Ferrari A, Elad Y, Pertot I (2008) Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol Control 47:228–234
Perez-Miranda S, Cabirol N, George-Tellez R, Zamudio-Rivera LS, Fernandez FJ (2007) O-CAS, a fast and universal method for siderophore production. J Microbiol Methods 70:127–131
Purwantisari S, Priyatmojo A, Sancayaningsih RP, Kasiamdari RS, Budihardjo K (2018) Systemic inducing resistance against late blight by applying antagonist Trichoderma viride. In journal of physics: conference series, IOP publishing, 1025-p. 012053
Sadasivam S, Manickam A (1996) Biochemical methods for agricultural sciences. New age international (P) ltd., New Delhi, India
Samuels GJ, Dodd SL, Lu B-S, Petrini O, Schroers H-J, Druzhinina IS (2006) The Trichoderma koningii aggregate species. Stud Mycol 56:67–133
Samuels GJ, Ismaiel A, Bon MC, De Respinis S, Petrini O (2010) Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102:944–966
Sawant IS, Sawant SD (2011) Integration of Trichoderma harzianum 5R with low dose of Sulphur dioxide generator sheet for control of postharvest decay of Tas-A-Ganesh (Vitis vinifera L.) during and after long duration low temperature storage. J Eco-friendly Agric 6:180–186
Sawant IS, Rajguru YR, Salunkhe VP, Wadkar PN (2012) Evaluation and selection of efficient isolates of Trichoderma species from diverse locations in India for biological control of anthracnose disease of grapes. J Biol Control 26:144–154
Sawant SD, Sawant IS, Shetty D, Shinde M, Jade S, Waghmare M (2011) Control of powdery mildew in vineyards by Milastin K, a commercial formulation of Bacillus subtilis (KTBS). J Biol Control 25:26–32
Sawant IS, Wadkar PN, Ghule SB, Rajguru YR, Salunkhe VP, Sawant SD (2017) Enhanced biological control of powdery mildew in vineyards by integrating a strain of Trichoderma afroharzianum with Sulphur. Biol Control 114:133–143
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87:787–799
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sharma P, Sain SK (2004) Induction of systemic resistance in tomato and cauliflower by Trichoderma spp. against stalk rot pathogen, Sclerotinia sclerotiorum (lib) de Bary. J Biol Control 18:21–28
Simko I, Piepho HP (2012) The area under the disease progress stairs: calculation, advantage, and application. Phytopathol 102:381–389
Singleton VL, Rossi JA (1965) Colorimetery of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am J Enol Vitic 19:144–158
Sumayo M, Ghim SY (2013) Plant growth-promoting rhizobacteria for plant immunity. In: Maheshwari D, Saraf M, Aeron A (eds) Bacteria in agrobiology: crop productivity. Springer, Berlin, Heidelberg
Vinale F, Nigro M, Sivasithamparam K, Flematti G, Ghisalberti EL, Ruocco M, Varlese R, Marra R, Lanzuise S, Eid A, Woo SL, Lorito M (2013) Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiol Lett 347:123–129. https://doi.org/10.1111/1574-6968.12231
Viterbo A, Horwitz B (2010) In: Borkovich K, Ebbole D (eds) Mycoparasitism. ASM Press, Cellular and Molecular Biology of Filamentous Fungi, pp 676–693
Viterbo A, Landau U, Kim S, Chernin L, Chet I (2010) Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol Lett 305:42–48
White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications eds. Innis MA, Gelfand DH, Sninsky JJ, White TJ (Academic Press, Inc, California), 315–322
Woo SL, Scala F, Ruocco M, Lorito M (2006) The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathol 96:181–185
Yedidia I, Benhamou N, Chet I (1999) Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 65:1061–1070
Yedidia I, Benhamou N, Kapulnik Y, Chet I (2000) Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol Biochem 38:863–873
Acknowledgements
The authors are thankful to the Indian Council of Agricultural Research (ICAR), New Delhi for funding the research under the AMAAS Project and Dr. A. K. Upadhyay for soil analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sawant, I.S., Wadkar, P.N., Ghule, S.B. et al. Induction of systemic resistance in grapevines against powdery mildew by Trichoderma asperelloides strains. Australasian Plant Pathol. 49, 107–117 (2020). https://doi.org/10.1007/s13313-020-00679-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00679-8