Abstract
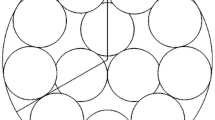
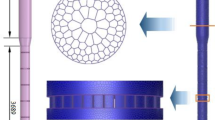
The synthesis of vinyl acetate (VAc) from ethylene is a strongly exothermic reaction that might easily cause catalyst deactivation and reduce selectivity of VAc. Research at the bed scale helps to improve the conversion of C2H4 and the selectivity of VAc. In this study, the discrete element method (DEM) was used to construct a fixed-bed structure model via simulating the filling process of catalyst particles in the reactor. The inlet section of a reaction tube was studied, and its length was 10 cm. The temperature distribution, and the effects of particles size, inlet velocity, inlet temperature and the feed ratio of C2H4 to O2 on the reaction process were studied. Simulated results show that the bed temperature gradually increased from the wall to the center, and the temperature gradient gradually decreased along the radial direction. The maximum temperature was 438.68 K and the temperature difference from the inlet temperature was 5.54 K. Comparing the composite particle packed bed with the single particle size packed bed, the composite packed bed has higher vinyl acetate selectivity. Increasing inlet velocity from 1.5 m/s to 3.5 m/s, the selectivity of vinyl acetate increased from 91.71% to 92.60%. Adding an inert gas to the feed gas can increase the oxygen concentration and reduce the explosion interval of QH4, the conversion of C2H4 and the selectivity of vinyl acetate increased.
Similar content being viewed by others
Abbreviations
- ∇:
-
divergence
- Cg :
-
specific heat capacity of vapor phase [J·kg−1 ·K−1]
- Cp :
-
specific heat capacity of fluid [J·kg−1 · K−1]
- Cs :
-
specific heat capacity of solid phase [J·kg−1 · K−1]
- dp :
-
particle diameter [m]
- dt :
-
tube diameter [m]
- C1ε C2ε C3e :
-
empirical constant
- Di :
-
diffusion coefficient of component i [m2sd-s−1]
- ECO2 :
-
the apparent activation energy of side reaction
- EVAC :
-
the apparent activation energy of main reaction
- F:
-
catalyst surface area [m2]
- G b :
-
the turbulent kinetic energy generation term caused by buoyancy gives rise
- G k :
-
the turbulent kinetic energy generation term caused by mean velocity gradient
- ΔH:
-
standard enthalpy of formation [kJ·mor−1]
- h:
-
the height of the bed [m]
- hd :
-
the section increased in the lower stage of the tube
- hg :
-
gas phase mass transfer coefficient [W/m−2·KT−1]
- hs :
-
solid phase mass transfer coefficient [W/m−2·KT−1]
- hu :
-
the section increased in the upper stage of the tube
- Mi :
-
mass fraction of component i
- N:
-
the tube-to-particle diameter ratio
- p̄’:
-
the average void ratio
- r1 :
-
reaction rate of main reaction [mol·g−1min−1]
- r2 :
-
reaction rate of side reaction [mol·g−1min−1]
- Rep :
-
particle Reynolds number
- Sκ :
-
turbulent kinetic energy source items [J]
- Sε>:
-
turbulent dissipation rate source term
- T:
-
temperature [°C]
- Tc :
-
the temperature of the volume near the wall [K]
- Tg :
-
the temperature of vapor phase [K]
- ūi :
-
mean velocity component of i direction [m-s−1]
- u’i :
-
fluctuation velocity of i direction [m-s−1]
- VAc:
-
vinyl acetate
- wi :
-
molar mass of component [kg·mor−1]
- yc :
-
the normal distance of the volume near the wall [m]
- YM :
-
contribution of pulsating expansion in compressible turbulence
- Κ :
-
turbulence kinetic energy [J]
- ε :
-
turbulent dissipation rate
- ρ :
-
density [kg·m−3]
- ν:
-
velocity [m·s−1]
- νin :
-
inlet velocity [m·s−1]
- µ :
-
dynamic viscosity [Pa·s]
- σε :
-
Prandtl number corresponding to the turbulent dissipation rate
- σΚ :
-
Prandtl number corresponding to turbulent kinetic energy
References
N. Panda and S. A. Yadav, Asian J. Chem., 8, 296 (2019).
H. F. Rase and M. Hayes, Platin Met. Rev., 45, 83 (2001).
S. Geng, M. U. Haque and K. Oksman, Compos. Sci. Technol, 126, 35 (2016).
T. V. Panova, A. A. Efimova and A. V. Efimov, Colloid Polym. Sci, 7, 1 (2019).
A. R. G. Caranton, J. Dille, J. Barreto, F. Stavale, J. C. Pinto and M. Schmal, ChemCatChem, 10, 5256 (2018).
Y. F. Han, J. H. Wang, D. Kumar, Z. Yan and D. W. Goodman, J. Catal, 232, 467 (2005).
Y. F. Han, D. Kumar, C. Sivadinarayana and D. W. Goodman, J. Catal, 224, 60 (2004).
X. Dong, Y. Wang, Y. Yu and M. H. Zhang, Ind. Eng. Chem. Res., 57, 7363 (2018).
K. Motahari, H. Atashi, F. Fazloflahi and M. Sarkari, find Eng. Chem., 18, 266 (2012).
K. Motahari, G. Rempel, S. Lashkarara, K. Ghaseminezhad, A. Boramandnejad and B. Hatami, Can. J. Chem. Eng, 94, 506 (2016).
D. Stacchiola, F. Calaza, M. Neurock and W. T. Tysoe, J. Am. Chem. Soc., 132, 2202 (2010).
S. Nakamura and T. Yasui, J. Catal, 54, 605 (1982).
Q. L. Pham, Y. Haldorai and V. H. Nguyen, Korean J. Chem. Eng, 31, 2101 (2014).
A. Talebian, A. R. Keshtkar and M. A. Mohammad, Korean J. Chem. Eng, 33, 2205 (2016).
S. A. Jafari and A. Jamali, Korean J. Chem. Eng, 33, 1296 (2016).
A. G. Dixon, M. Nijemeisland and E. H. Stitt, Adv. Chem. Eng, 31, 307 (2006).
S. A. Logtenberg and A. G. Dixon, Chem. Eng. Process., 37, 7 (1998).
P. X. Jiang, R. N. Xu and W. I. Gong, Chem. Eng. Sci., 61, 7213 (2006).
A. Guardo, M. Coussirat, M. A. Larrayoz, F. Recasens and E. Egus-quiza, Chem. Eng. Sci, 60, 1733 (2005).
P. R. Gunjal, V. V. Ranade and R. V. Chaudhari, AIChE J., 51, 365 (2005).
H. Atashi, M. Sarkari, K. Motahari, F. F. Tabrizi and F. Fazlollahi, J. Korean Chem. Soc., 55, 92 (2011).
K. Motahari, G. Rempel, S. Lashkarara, K. Ghaseminezhad, A. Borumandnejad and B. Hatami, Can. J. Chem. Eng, 94, 506 (2016).
Y. F. Han, D. Kumar and D. W. Goodman, J. Catal., 230, 353 (2005).
B. Partopour, AIChE J., 63, 87 (2017).
B. Partopour and A. G. Dixon, Ind. Eng. Chem. Res., 55, 7296 (2016).
D. Ying, F. J. Keil, O. Korup, F. Rosowski and R. Horn, Chem. Eng. Sci, 142, 299 (2016).
R. Y. Hong, W. Yang, Y. Q. Zhuang and H. Z. Li, Comput. A. Chem., 23, 481 (2006).
P. Behnam and A. G. Dixon, AIChE J.63, 87 (2017).
G. D. Wehinger, M. Kraume, V. Berg, O. Korup, K Metre, R. Schlogl, M. Behrens and R. Horn, AIChE J., 62, 4436 (2016).
G. D. Wehinger, T. Eppinger and M. Kraume, Chem. Eng. Sci, 122, 197 (2015).
K. Vollmari, T. Oschmann, S. Wirtz and H. K. Emden, Powder Technol, 271, 109 (2015).
X. M. Zhou, Y.J. Duan, X. L. Huai and X. E. Li, Particuology, 11, 715 (2012).
V. Mandar, S. T. Johansen and S. Amini, Ind. Eng. Chem. Res., 52, 12041 (2013).
A. G. Dixon, Can. J. Chem. Eng, 66, 705 (1988).
G. D. Wehinger, T. Eppinger and M. Kraume, Chem. Eng. Sci, 122, 197 (2015).
A. G. Dixon, M. Nijemeisland and E. H. Stitt, Comput. Chem. Eng, 48, 135 (2013).
S. H. Cheng, H. Chang and Y. H. Chen, Computational fluid dynamics-based multiobjective optimization for catalyst design, 21st International Symposium on Chemical Reaction Engineering, Philadelphia, PA, 13–16 July (2010).
Z. X. Zhao, Q. L. Dai, S. D. Wang, B. Y. Lin and G. T. Chen, Chem. React. Eng. Technol, 2, 128 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Wang, M., Cao, X. et al. Particle resolved CFD simulation on vapor-phase synthesis of vinyl acetate from ethylene in fixed-bed reactor. Korean J. Chem. Eng. 37, 839–849 (2020). https://doi.org/10.1007/s11814-020-0500-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0500-y