Abstract
The reaction of perfluoropyridines with indoles and bis(indolyl)methanes (BIMs) was investigated. The aromatic nucleophilic substitution of pentafluoropyridine with indoles occurs at the 4-position of pyridine ring by nitrogen site of indolyl anion, while reaction with 2,3,5,6-tetrafluoro-4-(phenylsulfonyl)pyridine gave a mixture of products arising substitution at 2-position of pyridine ring and replacement of phenylsulfonyl group. Furthermore, the reaction of pentafluoropyridine with BIMs yielded mixture of mono- and bis-perfluoropyridyl products.
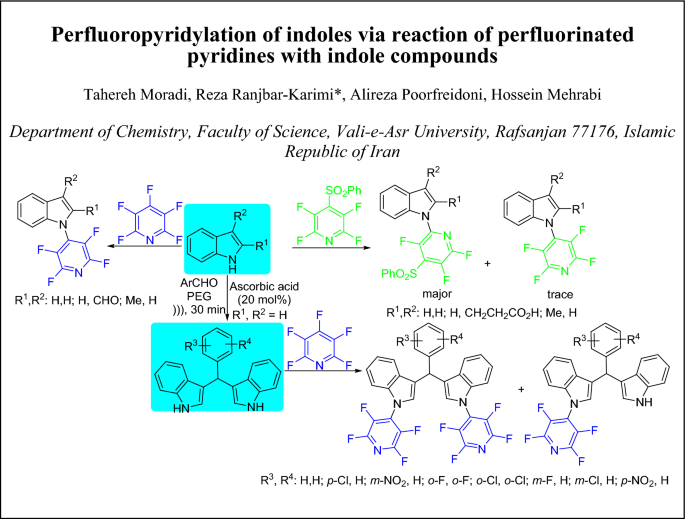
Graphic abstract



Similar content being viewed by others
References
I.V. Trushkov, M.G. Uchuskin, V.T. Abaev, O.V. Serdyuk, Synthesis 51, 787 (2019)
I. Chen, S. Safe, L. Bjeldanes, Biochem. Pharmacol. 51, 1069 (1996)
D. Kumar, S. Sundaree, E.O. Johnson, K. Shah, Bioorg. Med. Chem. Lett. 19, 4492 (2009)
D. Kumar, N.M. Kumar, S. Sundaree, E.O. Johnson, K. Shah, Eur. J. Med. Chem. 45, 1244 (2010)
D. Kumar, N.M. Kumar, K.H. Chang, K. Shah, Eur. J. Med. Chem. 45, 4664 (2010)
D. Kumar, M.K. Narayanam, K.H. Chang, K. Shah, Chem. Biol. Drug Des. 77, 182 (2011)
I. Hatti, R. Sreenivasulu, S.S. Jadav, M.J. Ahsan, R.R. Raju, Monatsh. Chem. 146, 1699 (2015)
S. Suzen, E. Buyukbingol, II Farmaco 55, 246 (2000)
G. Giagoudakis, S.L. Markantonis, Pharmacotherapy 25, 18 (2005)
E. Buyukbingol, S. Suzen, G. Klopman, Farmaco 49, 443 (1994)
S. Suzen, E. Buyukbingol, II Farmaco 53, 525 (1998)
G. Bartoli, R. Dalpozzo, M. Nardi, Chem. Soc. Rev. 43, 4728 (2014)
Z.R. Owczarczyk, W.A. Braunecker, A. Garcia, R. Larsen, A.M. Nardes, N. Kopidakis, D.S. Ginley, D.C. Olson, Macromolecules 46, 1350 (2013)
G. Nie, Z. Bai, W. Yu, L. Zhang, J. Polym. Sci. Part A Polym. Chem. 51, 2385 (2013)
L. Gupta, A. Talwar, P.M.S. Chauhan, Curr. Med. Chem. 14, 1789 (2007)
G.L. Tolnai, A. Szekely, Z. Mako, T. Gati, J. Daru, T. Bihari, A. Stirling, Z. Novak, Chem. Commun. 51, 4488 (2015)
S.J. Yao, Z.H. Ren, Y.Y. Wang, Z.H. Guan, J. Org. Chem. 81, 4426 (2016)
J.B. Thomas, A.M. Giddings, R.W. Wiethe, S. Olepu, K.R. Warner, P. Sarret, L. Gendron, J.M. Longpre, Y. Zhang, S.P. Runyon, B.P. Gilmour, J. Med. Chem. 57, 7472 (2014)
Y. Gao, S. Osman, K. Koide, A.C.S. Med, Chem. Lett. 5, 863 (2014)
C.H. Ma, T.R. Kang, L. He, Q.Z. Liu, Eur. J. Org. Chem. 19, 3981 (2014)
T. Kawanami, A.S. Karns, C.M. Adams, M. Serrano-Wu, Tetrahedron Lett. 54, 7202 (2013)
A.S. Rowan, T.S. Moody, R.M. Howard, T.J. Underwood, I.R. Miskelly, Y. He, B. Wang, Tetrahedron Asymmetry 24, 1369 (2013)
J. Pedroni, N. Cramer, Org. Lett. 18, 1932 (2016)
M. Baumann, I.R. Baxendale, S.V. Ley, N. Nikbin, Beilstein J. Org. Chem. 7, 442 (2011)
I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Ch. 11 (Wiley, Hoboken, 2009)
V.A. Petrov, Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, Ch. 8 (Wiley, Hoboken, 2009)
V. Nenajdenko, Fluorine in Heterocyclic Chemistry Volume 1: 5-Membered Heterocycles and Macrocycles, vol. 2 (Springer, Berlin, 2014)
H.P. Ng, B.O. Buckman, K.A. Eagen, W.J. Guilford, M.J. Kochanny, R. Mohan, K.J. Shaw, S.C. Wu, D. Lentz, A. Liang, L. Trinh, E. Ho, D. Smith, B. Subramanyam, R. Vergona, J. Walters, K.A. White, M.E. Sullivan, M.M. Morrissey, G.B. Phillips, Bioorg. Med. Chem. 3, 657 (2002)
L. Revesz, F.E. Di Padova, T. Buhl, R. Feifel, H. Gram, P. Hiestand, U. Manning, R. Wolf, A.G. Zimmerlin, Bioorg. Med. Chem. Lett. 12, 2109 (2002)
R.D. Chambers, A. Khalil, C.B. Murray, G. Sandford, A.S. Batsanov, J.A.K. Howard, J. Fluorine Chem. 126, 1002 (2005)
A. Baron, G. Sandford, R. Slater, D.S. Yufit, J.A.K. Howard, A. Vong, J. Org. Chem. 70, 9377 (2005)
R.D. Chambers, P.R. Hoskin, A.R. Kenwright, A. Khalil, P. Richmond, G. Sandford, D.S. Yufit, J.A. Howard, Org. Biomol. Chem. 1, 2137 (2003)
R. Ranjbar-Karimi, A. Poorfreidoni, H.R. Masoodi, J. Fluorine Chem. 180, 222 (2015)
R. Ranjbar-Karimi, A. Darehkordi, F. Bahadornia, A. Poorfreidoni, J. Heterocycl. Chem. 55, 2516 (2018)
A. Poorfreidoni, R. Ranjbar-Karimi, Tetrahedron Lett. 57, 5781 (2016)
R. Ranjbar-Karimi, A. Karbakhsh-Ravari, A. Poorfreidoni, J. Iran. Chem. Soc. 14, 2397 (2017)
R. Ranjbar-Karimi, E. Heidari, J. Fluorine Chem. 154, 47 (2013)
R. Ranjbar-Karimi, M. Mousavi, J. Fluorine Chem. 131, 587 (2010)
J.A. Joule, K. Mills, Heterocyclic Chemistry, Ch. 20, 5th edn. (Wiley, Hoboken, 2010)
M.W. Cartwright, G. Sandford, J. Bousbaa, D.S. Yufit, J.A. Howard, J.A. Christopher, D.D. Miller, Tetrahedron 63, 7027 (2007)
M.W. Cartwright, L. Convery, T. Kraynck, G. Sandford, D.S. Yufit, J.A. Howard, J.A. Christopher, D.D. Miller, Tetrahedron 66, 519 (2010)
S.R. Mendes, S. Thurow, F. Penteado, M.S. da Silva, R.A. Gariani, G. Perin, E.J. Lenardao, Green Chem. 17, 4334 (2015)
S.R. Mendes, S. Thurow, M.P. Fortes, F. Penteado, E.J. Lenardao, D. Alves, G. Perin, R.G. Jacob, Tetrahedron Lett. 53, 5402 (2012)
N.C. Ganguly, P. Mondal, S.K. Barik, Green Chem. Lett. Rev. 5, 73 (2012)
H. Veisi, B. Maleki, F. Hosseini Eshbala, H. Veisi, R. Masti, S.S. Ashrafi, M. Baghayeri, RSC Adv. 4, 30683 (2014)
S.S. Sonar, S.A. Sadaphal, A.H. Kategaonkar, R.U. Pokalwar, B.B. Shingate, M.S. Shingare, Bull. Korean Chem. Soc. 30(825), 825 (2009)
R. Surasani, D. Kalita, K.B. Chandrasekhar, Green Chem. Lett. Rev. 6, 113 (2013)
D. Talukdar, A.J. Thakur, Green Chem. Lett. Rev. 6, 55 (2013)
Y.L. Yang, N.N. Wan, W.P. Wang, Z.F. Xie, J.D. Wang, Chin. Chem. Lett. 22, 1071 (2011)
S.J. Ji, M.F. Zhou, D.G. Gu, Z.Q. Jiang, T.P. Loh, Eur. J. Org. Chem. 7, 1584 (2004)
B.S. Liao, J.T. Chen, S.T. Liu, Synthesis 20, 3125 (2007)
Acknowledgements
The authors wish to thank Vali-e-Asr University of Rafsanjan for partially funding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moradi, T., Ranjbar-Karimi, R., Poorfreidoni, A. et al. Perfluoropyridylation of indoles via reaction of perfluorinated pyridines with indole compounds. J IRAN CHEM SOC 17, 1347–1357 (2020). https://doi.org/10.1007/s13738-020-01858-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01858-6




