Abstract
Key message
MsCBL4 expression in tobacco enhanced its salt and saline–alkali stress tolerance by regulating calcium accumulation in roots, indicating the important role of calcium metabolism in plant saline–alkali stress tolerance
Abstract
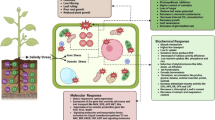
The calcineurin B–like (CBL) family of proteins play important roles in plant abiotic stress tolerance and signal transduction. CBL4 is known to participate in the Salt Overly Sensitive pathway; however, little is currently known regarding the mechanisms underlying the response of CBL4 to saline–alkali stress. In this study, we cloned and characterized the alfalfa MsCBL4 gene. We found that MsCBL4 showed the highest expression in root tissues and was induced by salt and saline–alkali stress, with the latter causing higher induction. Overexpression of MsCBL4 in tobacco enhanced salt and saline–alkali stress tolerance and reduced the Na+/K+ ratio in roots of transgenic lines. Salt (30 and 300 mM NaCl) and saline–alkali (30 mM NaHCO3) stress assays performed for MsCBL4 transgenic tobacco lines revealed a substantial influx of sodium ions in roots under saline–alkali stress and indicated that the expression of MsCBL4 had little influence on sodium ion content reduction. In contrast, in roots subjected to saline–alkali stress, calcium accumulation occurred and was significantly enhanced by the overexpression of MsCBL4. Physiological and biochemical analyses indicated that MsCBL4 plays an important role in saline–alkali stress tolerance via its influence on the regulation of calcium transport and accumulation. These results provide novel insights into the saline–alkali stress tolerance mechanisms of plants.










Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
An YM, Song LL, Liu YR, Guo CH et al (2016) De novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front Plant Sci 7:931
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bergmeyer HU, Bergmeyer J, Grassl M (1974) Methods of enzymatic analysis. Vol. 2, Samples, reagents, assessment of results[M]. Verlag Chemie
Bertorello AM, Zhu JK (2009) SIK1/SOS2 networks: decoding sodium signals via calcium-responsive protein kinase pathways. Pfluegers Arch 458(3):613–619
Bickerton P, Pittman J (2015) Role of Cation/Proton Exchangers in Abiotic Stress Signaling and Stress Tolerance in Plants. Elucidation of Abiotic Stress Signaling in Plants: 95–177
Caland LB, Silveira ELC, Tubino M (2011) Determination of sodium, potassium, calcium and magnesium cations in biodiesel by ion chromatography. Anal Chim Acta 718:116–120
Chen K, Kurgan L, Rahbari M (2007) Prediction of protein crystallization using collocation of amino acid pairs. Biochem Biophys Res Commun 335:764–769
Chen L, Ren J, Shi H, Peng J et al (2015) TdCBL6, a calcineurin B-like gene from wild emmer wheat (Triticum dicoccoides), is involved in response to salt and low-K+ stresses. Mol Breed 35(1):35–50
Dong LH, Qian W, Manik S, Liu HB et al (2015) Nicotiana sylvestris calcineurin B-like protein NsylCBL10 enhances salt tolerance in transgenic Arabidopsis. Plant Cell Rep 34:2053–2063
El-Gebali S, Mistry J, Bateman A et al (2019) The Pfam protein families database in 2019: Finn. Nucleic Acids Res 47:D427–D432
Gong B, Wen D, Vandenlangenberg K, Wang XF et al (2013) Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic 157:1–12
Guo R, Yang ZZ, Li F, Yan CR, Zhong XL, Zhao L et al (2015) Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol 15:170
Hadi MR, Karimi N (2011) The role of calcium in plant salt tolerance. J Plant Nutr 35:2037–2054
Hadi MR, Khiyam-Nekoie KR, Yavari P et al (2008) Accumulation and role of ions (Ca2+, Mg2+, SO42-) on salt tolerance in Triticum turgidum L. J Biol Sci 8(1):143–148
Held K, Pascaud F, Kudla J et al (2011) Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. https://doi.org/10.1038/cr.2011.50
Hobbs SL, Kpodar P, DeLong CM (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hu LX, Zhang PP, Jiang Y, Fu JM (2015) Metabolomic analysis revealed differential adaptation to salinity and alkalinity stress in kentucky bluegrass (Poa pratensis). Plant Mol Biol Rep 33:56–68
Kang HK, Nam KH (2016) Reverse function of ROS-induced CBL 10 during salt and drought stressresponses. Plant Sci 243:49–55
Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J (2004) Calcium sensors and their interacting protein kinases: genomics of the arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol 134:43–58
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Li RL, Shi FC, Fukuda KJ, Yang YL (2011) Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci Plant Nutr 56(5):725–733
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Biol Ecol 382:82–87
Liu JY, Niu YF, Zhang JJ, Huang X et al (2018) Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: recent advances. Plant Cell Tissue Organ Cult 132:413–424
Liu LL, Ren HM, Chen LQ, Wang Y, Wu WH (2013) A protein kinase, calcineurin B-like protein-interacting protein kinase 9, interacts with calcium sensor calcineurin B-like protein3 and regulates potassium homeostasis under low-potassium stress in arabidopsis. Plant Physiol 161(1):266–277
Ma DM, Xu WD, Li HW, Xu X et al (2014) Co-expression of the Arabidopsis SOS genes enhances salt tolerance in transgenic tall fescue (Festuca arundinacea Schreb.). Protoplasma 251:219–231
Mahajan S, Pandey G, Tuteja N (2008) Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch Biochem Biophys 471(2):146–158
Meneguzzo S, Navari-Izzo F, Izzo R (2000) NaCI effects on water relations and accumulation of mineral nutrients in shoots, roots and cell sap of wheat seedlings. J Plant Physiol 156:711–716
Midhat U, Ting MKY, Teresinski HJ, Snedden WA (2018) The calmodulin-like protein, CML39, is involved in regulating seed development, germination, and fruit development in Arabidopsis. Plant Mol Biol https://doi.org/10.1007/s11103-018-0703-3
Mouttet P, Escobar-Gutiérrez A, Esquibet M et al (2014) Banning of methyl bromide for seed treatment: could Ditylenchus dipsaci again become a major threat to alfalfa production in Europe. Pest Manag Sci 70(7):1017–1022
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Plant Physiol 58:166–170
Oosten M, Sharkhuu A, Batelli G, Bressan R, Maggio A (2013) The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol Biol 83:405–415
Pandey GK, Kanwar P, Luan S et al (2015) Calcineurin B-Like protein-interacting protein kinase CIPK21 regulates osmotic and salt stress responses in arabidopsis. Plant Physiol 169:780–792
Passonneau JV, Lowry OH (1993) Enzymatic analysis: a practical guide. Springer Science & Business Media, Berlin
Peng YL, Gao ZW, Gao Y, Wang DL et al (2008) Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J Integr Plant Biol 50(1):29–39
Rodríguez-Rosales MP, Galvez FJ, Venema K et al (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4(4):265–276
Sanchez-Barrena MJ, Martinez-Ripoll M, Zhu JK, Albert A (2005) The structure of the arabidopsis thaliana SOS3: molecular mechanism of sensing calcium for salt stress response. J Mol Biol 345(5):1253–1264
Sathee L, Sairam RK, Chinnusamy V, Jha SK (2015) Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiologiae Plantarum. https://doi.org/10.1007/s11738-015-1910-z
Shi HZ, Kin YS, Guo Y, Stevenson B, Zhu JK (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15:19–32
Shi HZ, Xiong LM, Stevenson B, Lu T, Zhu JK (2002) The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for Vitamin B6 in plant salt tolerance. Plant Cell 14:575–588
Shi J, Fu XZ, Peng T, Liu JH et al (2009) Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol 30:914–922
Shukla PS, Agarwal PK, Jha B (2012) Improved salinity tolerance of arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J Plant Growth Regul 31:195–206
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Viladevall L, Serrano R, Ruiz A, Domenech G, Arino J et al (2004) Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem 279(42):43614–43624
Wang H, Liang Y, Zhang B, Zheng W, Xing LJ, Li MC (2011) Alkaline stress triggers an immediate calcium fuctuation in Candida albicans mediated by Rim101p andCrz1p transcription factors. Yeast Res. https://doi.org/10.1111/j.1567-1364.2011.00730.x
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wu WH, Wang Y, Lee SC, Lan WZ, Luan S (2010) Regulation of ion channels by the calcium signaling network in plant cells. Signal Commun Plants. https://doi.org/10.1007/978-3-642-10494-7_6
Xiong LM, Schumaker K, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:165–183
Yadav NS, Shukla PS, Jha A, Agarval PK, Jha B (2012) The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol 12:188
Yang CW, Guo WQ, Shi DC (2010) Physiological roles of organic acids in alkali-tolerance of the alkali-tolerant halophyte Chloris virgata. Agron J 102(4):1081–1089
Yang Q, Chen ZZ, Zhou XF, Gong ZZ et al (2009) Overexpression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic arabidopsis. Molecular Plant 2(1):22–31
Yang YQ, Wu YJ, Ma L et al (2019) The Ca2+ sensor SCaBP3/CBL7 modulates plasma membrane H+-ATPase activity and promotes alkali tolerance in arabidopsis. Plant Cell 31(6):1367–1384
Zhang CX, Bian MD, Yu H, Liu Q, Yang ZM (2011) Identification of alkaline stress-responsive genes of CBL family in sweet sorghum (Sorghum bicolor L.). Plant Physiol Biochem 49:1306–1312
Zhang XG, Huang B, Hu WY et al (2013) Study on salinization characteristics of surface soil in western songnen plain. Soil 45(2):332–338
Zhang YM, Linghu JJ, Wang D, Zhao TY et al (2017) Foxtail millet CBL4 (SiCBL4) interacts with SiCIPK24, modulates plant salt stress tolerance. Plant Mol Biol Rep 35:634–646
Zhao YK, Wang T, Zhang WS, Li X (2011) SOS3 mediates lateral root development under low salt stress through regulation of auxin redistribution and maxima in Arabidopsis. New Phytol 189:1122–1134. https://doi.org/10.1111/j.1469-8137.2010.03545.x
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu S, Zhou XP, Wu XM, Jiang ZH (2013) Structure and function of the CBL–CIPK Ca2+-decoding system in plant calcium signaling. Plant Mol Biol Rep 31:1193–1202
Acknowledgements
This work was supported by grants from the National and Science Foundation of China (grant numbers 31470571, 31972507); the National Major Project for Cultivation of Transgenic Crops (grant number 2016ZX08004-002-003); the National Key Research and Development Program of China (grant number 2017YFD0101303). Science and Technology Major Project of Heilongjiang Province (GA18B104); Science and Technology Achievement Cultivation Project of Heilongjiang Education Department (TSTAU-R2018008); Graduate Innovation Fund of Harbin Normal University (HSDSSCX2019-23); Research Fund of Beijing Advanced Innovation Center for Food Nutrition and Human Health (20182018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Günther Hahne.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
An, Y., Yang, XX., Zhang, L. et al. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep 39, 997–1011 (2020). https://doi.org/10.1007/s00299-020-02543-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02543-x