Abstract
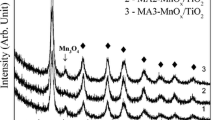
The low-temperature catalytic NO removal efficiencies of catalysts with two different manganese precursors, manganese acetate (αMA catalyst) and manganese nitrate (αMN catalyst), were compared and studied. The NH3-SCR of NO tests using these catalysts were carried out in a self-made reaction device to measure the catalytic activity of the catalysts. Moreover, the reaction pathway analysis of NH3-SCR of NO on the surface of MnO2/SiO2 catalyst and Mn2O3/SiO2 catalyst were performed based on DFT calculations individually. The experimental results show that the NO conversion of αMN catalyst is higher than the αMA catalyst at low temperature (< 180 °C). Besides, the XRD test shows that the main crystal phase is MnO2 for αMN catalysts, and Mn2O3 for αMA catalyst, and the XPS characterization exhibits that the αMN catalyst has the highest MnO2/Mn2O3 ratio. Moreover, DFT calculations indicate that the decompositions of NH2NO and NHNO are the rate determining steps in the whole NH3-SCR of NO process and the decomposition activation energy of NH2OH and NHNO on MnO2 is lower than that on Mn2O3. This is the main reason for the higher NO conversion of catalyst prepared with manganese nitrate as precursor at low temperature.









Similar content being viewed by others

References
Busca G, Lietti L, Ramis G, Berti F (1998) Appl Catal B 18:1–36
Busca G, Larrubia MA, Arrighi L, Ramis G (2005) Catal Today 107–108:139–148
Wallin M, Forser S, Thormählen P, Skoglundh M (2004) Ind Eng Chem Res 43:7723–7731
Peña DA, Uphade BS, Smirniotis PG (2004) J Catal 221:421–431
Martin D, Duprez D (1997) J Phys Chem B 101:4428–4436
Martin D, Duprez D (1996) J Phys Chem 100:9429–9438
Zhang L, Qu H, Du T, Ma W, Zhong Q (2016) Chem Eng J 296:122–131
Jing W, Guo Q, Hou Y, Han X, Huang Z (2014) Korean J Chem Eng 31:794–800
Xiao X, Sheng Z, Yang L, Dong F (2016) Catal Sci Technol 6:1507–1514
Lu X, Song C, Jia S, Tong Z, Tang X, Teng Y (2015) Chem Eng J 260:776–784
Zuo J, Chen Z, Wang F, Yu Y, Wang L, Li X (2014) Ind Eng Chem Res 53:2647–2655
Lee SM, Park KH, Kim SS, Kwon DW, Hong SC (2012) J Air Waste Manag Assoc 62:1085–1092
Li J, Chang H, Ma L, Hao J, Yang RT (2011) Catal Today 175:147–156
Liu C, Shi J, Gao C, Niu C (2016) Appl Catal A: Gen 522:54–69
Smirniotis PG, Sreekanth PM, Peña DA, Jenkins RG (2006) Ind Eng Chem Res 45:6436–6443
Huang J, Tong Z, Huang Y, Zhang J (2008) Appl Catal B 78:309–314
Kapteijn F, Vanlangeveld AD, Moulijn JA, Andreini A, Vuurman MA, Turek AM, Jehng JM, Wachs IE (1994) J Catal 150:94–104
Peng Y, Chang H, Dai Y, Li J (2013) Procedia Environ Sci 18:384–390
Guo P, Guo X, Zheng C (2010) Appl Surf Sci 256:6991–6996
Zhao Q, Liu Y, Xiang J, Sun L, Su S, Hu S (2010) DFT Study of NH3 and NO Adsorption On Copper-Aluminate Catalysts, IEEE, 2010, pp 1–4.
Delley B (2000) J Chem Phys 113:7756–7764
Hu Z, Turner CH (2006) J Phys Chem B 110:8337–8347
Ren D, Gui K (2019) Appl Surf Sci 487:171–179
Xiang J, Wang L, Cao F, Qian K, Su S, Hu S, Wang Y, Liu L (2016) Chem Eng J 302:570–576
Yang Y, Liu J, Wang Z, Liu F (2018) Fuel Process Technol 174:17–25
Liu Z, Zhu J, Li J, Ma L, Woo SI (2014) Acs Appl Mater Interface 6:14500–14508
Liu J, Li X, Zhao Q, Ke J, Xiao H, Lv X, Liu S, Tadé M, Wang S (2017) Appl Catal B 200:297–308
Rozanska X, Delbecq F, Sautet P (2010) Phys Chem Chem Phys 12:14930
Simonetti S, Compañy AD, Brizuela G, Juan A (2016) Colloid Surf B 148:287–292
Zhang L, Cui S, Guo H, Ma X, Lu W (2016) Comp Mater Sci 112:238–244
Xie C, Yang S, Shi J, Niu C (2019) React Kinet Mech Cat 128:681–693
Yang Y, Liu J, Liu F, Wang Z, Ding J, Huang H (2019) Chem Eng J 361:578–587
Gao F, Tang X, Yi H, Zhao S, Li C, Li J, Shi Y, Meng X (2017) Catalysts 7:199
Deng S, Zhuang K, Xu B, Ding Y, Yu L, Fan Y (2016) Catal Sci Technol 6:1772–1778
Metkar PS, Salazar N, Muncrief R, Balakotaiah V, Harold MP (2011) Appl Catal B 104:110–126
Savara A, Li M, Sachtler WMH, Weitz E (2008) Appl Catal B 81:251–257
Ciardelli C, Nova I, Tronconi E, Chatterjee D, Bandl-Konrad B (2004) Chem Commun 23:2718
Wang D, Zhang L, Kamasamudram K, Epling WS (2013) Acs Catal 3:871–881
Boningari T, Pavani SM, Ettireddy PR, Chuang SSC, Smirniotis PG (2018) Mol Catal 451:33–42
Acknowledgements
Financial support for this project from the National Nature Science Foundation of China (51276039) and the Research Subject of Environmental Protection Department of Jiangsu Province of China (2015008) are gratefully acknowledgment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, S., Gui, K., Ren, D. et al. The effects of manganese precursors on NO catalytic removal with MnOx/SiO2 catalyst at low temperature. Reac Kinet Mech Cat 130, 195–215 (2020). https://doi.org/10.1007/s11144-020-01772-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01772-1