Abstract
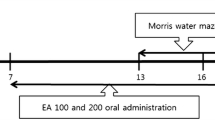
Amyloid β (Aβ) peptides produced from the amyloid precursor protein, a transmembrane protein, are neurotoxic and blocking the neurotoxicity may lead to prevention of Alzheimer’s disease (AD). Here we tested whether Aβ25–35-induced cognitive decline is rescued by treatment with dehydroeffusol, a phenanthrene isolated from Chinese medicine Juncus effusus. Dehydroeffusol (5 ~ 15 mg/kg body weight) was orally administered to mice for 6 days and Aβ25–35 (2 mM) was injected at the rate of 1 μl/min for 3 min into the lateral ventricle. Y-maze test was performed after dehydroeffusol administration for 12 days. Aβ25–35 impaired learning and memory in the test, while the impairment was dose-dependently rescued by dehydroeffusol administration. The present study indicates that treatment with dehydroeffusol is effective for rescuing Aβ25–35-induced cognitive decline.


Similar content being viewed by others
References
Morrison JH, Hof PR (1997) Life and death of neurons in the aging brain. Science 278:412–419. https://doi.org/10.1126/science.278.5337.412
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. https://doi.org/10.3233/jad-2001-3509
Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet 19:R12–R20. https://doi.org/10.1093/hmg/ddq160
Schoonenboom NS, Mulder C, Van Kamp GJ, Mehta SP, Scheltens P, Blankenstein MA, Mehta PD (2005) Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol 58:139–142. https://doi.org/10.1002/ana.20508
Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G (2000) High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058. https://doi.org/10.1523/JNEUROSCI.20-11-04050.2000
Selkoe DJ (2008) Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res 192:106–113. https://doi.org/10.1016/j.bbr.2008.02.016
Millucci L, Ghezzi L, Bernardini G, Santucci A (2010) Conformations and biological activities of amyloid β peptide 25−35. Curr Protein Pept Sci 11:54–67. https://doi.org/10.2174/138920310790274626
Smith AK, Klimov DK (2018) Binding of cytotoxic Aβ25-35 peptide to the dimyristoylphosphatidylcholine lipid bilayer. J Chem Inf Model 58:1053–1065. https://doi.org/10.1021/acs.jcim.8b00045
Clementi ME, Marini S, Coletta M, Orsini F, Giardina B, Misiti F (2005) Aβ(31−35) and Aβ(25−35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: role of the redox state of methionine-35. FEBS Lett 579:2913–2918. https://doi.org/10.1016/j.febslet.2005.04.041
Tsai HHG, Lee JB, Shih YC, Wan L, Shieh FK, Chen CY (2014) Location and conformation of amyloid β(25−35) peptide and its sequence-shuffled peptides within membranes: implications for aggregation and toxicity in PC12 cells. ChemMedChem 9:1002–1011. https://doi.org/10.1002/cmdc.201400062
Wang YX, Xia ZH, Jiang X, Li LX, An D, Wang HG, Heng B, Liu YQ (2019) Genistein inhibits Aβ25-35-induced neuronal death with changes in the electrophysiological properties of voltage-gated sodium and potassium channels. Cell Mol Neurobiol 39:809–822. https://doi.org/10.1111/bcpt.13279
Pike C, Burdick D, Walencewicz A, Glabe C, Cotman C (1993) Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13:1676–1687. https://doi.org/10.1523/JNEUROSCI.13-04-01676.1993
Crichton GE, Bryan J, Murphy KJ (2013) Dietary antioxidants, cognitive function and dementia--a systematic review. Plant Foods Hum Nutr 68:279–292. https://doi.org/10.1007/s11130-013-0370-0
Liao YJ, Zhai HF, Zhang B, Duan TX, Huang JM (2011) Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice. Planta Med 77:416–420. https://doi.org/10.1055/s-0030-1250517
Wang Y, Wang Y, Zhai H, Liao Y, Zhang B, Huang J (2012) Phenanthrenes from Juncus effusus with anxiolytic and sedative activities. Nat Prod Res 26:1234–1239. https://doi.org/10.1080/14786419.2011.561491
Greca MD, Fiorentino A, Monaco P, Pinto G, Pollio A, Previtera L (1996) Action of antialgal compounds from Juncus effusus L. on Selenastrum capricornutum. J Chem Ecol 22:587–603. https://doi.org/10.1007/BF02033657
Singhuber J, Baburin I, Khom S, Zehl M, Urban E, Hering S, Kopp B (2012) GABA(a) receptor modulators from the Chinese herbal drug Junci Medulla--the pith of Juncus effusus. Planta Med 78:455–458. https://doi.org/10.1055/s-0031-1298174
Tamano H, Sato Y, Takiguchi M, Murakami T, Fukuda T, Kawagishi H, Suzuki M, Takeda A (2019) CA1 LTP attenuated by corticosterone is canceled by effusol via rescuing intracellular Zn2+ dysregulation. Cell Mol Neurobiol 39:975–983. https://doi.org/10.1007/s10571-019-00693-5
Lu P, Mamiya T, Lu LL, Mouri A, Niwa M, Hiramatsu M, Zou LB, Nagai T, Ikejima T, Nabeshima T (2009) Silibinin attenuates amyloid beta(25-35) peptide-induced memory impairments: implication of inducible nitric-oxide synthase and tumor necrosis factor-alpha in mice. J Pharmacol Exp Ther 331:319–326. https://doi.org/10.1124/jpet.109.155069
Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, Zou LB, Nabeshima T (2012) Xanthoceraside attenuates amyloid β peptide25–35-induced learning and memory impairments in mice. Psychopharmacology 219:181–190. https://doi.org/10.1007/s00213-011-2386-1
Hiramatsu M, Takiguchi O, Nishiyama A, Mori H, Hiramatsu M, Takiguchi O, Nishiyama A, Mori H (2010) Cilostazol prevents amyloid β peptide(25-35)-induced memory impairment and oxidative stress in mice. Br J Pharmacol 161:1899–1912. https://doi.org/10.1111/j.1476-5381.2010.01014.x
Aminyavari S, Zahmatkesh M, Farahmandfar M, Khodagholi F, Dargahi L, Zarrindast MR (2019) Protective role of Apelin-13 on amyloid β25-35-induced memory deficit; involvement of autophagy and apoptosis process. Prog Neuropsychopharmacol Biol Psychiatry 89:322–334. https://doi.org/10.1016/j.pnpbp.2018.10.00
Ghumatkar PJ, Patil SP, Peshattiwar V, Vijaykumar T, Dighe V, Vanage G, Sathaye S (2019) The modulatory role of phloretin in Aβ25-35 induced sporadic Alzheimer's disease in rat model. Naunyn Schmiedeberg's Arch Pharmacol 392:327–339. https://doi.org/10.1007/s00210-018-1588-z
Hynd MR, Scott HL, Dodd PR (2010) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int 45:583–595. https://doi.org/10.1016/j.neuint.2004.03.007
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542. https://doi.org/10.1007/s00424-010-0809-1
Lipton SA (2004) Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders. J Alzheimers Dis 6:S61–S74. https://doi.org/10.3233/jad-2004-6s610
Wang R, Reddy PH (2017) Role of glutamate and NMDA receptors in Alzheimer's disease. J Alzheimers Dis 57:1041–1048. https://doi.org/10.3233/JAD-160763
Inoue Y, Ueda M, Masuda T, Misumi Y, Yamashita T, Ando Y (2019) Memantine, a noncompetitive N-methyl-D-aspartate receptor antagonist, attenuates cerebral amyloid angiopathy by increasing insulin-degrading enzyme expression. Mol Neurobiol 56:8573–8588. https://doi.org/10.1007/s12035-019-01678-7
Gray CW, Patel AJ (1995) Neurodegeneration mediated by glutamate and beta-amyloid peptide: a comparison and possible interaction. Brain Res 691:169–179. https://doi.org/10.1016/0006-8993(95)00669-h
de Ceballos ML, Brera B, Fernández-Tomé MP (2001) Beta-amyloid-induced cytotoxicity, peroxide generation and blockade of glutamate uptake in cultured astrocytes. Clin Chem Lab Med 39:317–318. https://doi.org/10.1515/CCLM.2001.049
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Fukuda, T., Sato, Y., Takiguchi, M. et al. Dehydroeffusol Rescues Amyloid β25–35-Induced Spatial Working Memory Deficit. Plant Foods Hum Nutr 75, 279–282 (2020). https://doi.org/10.1007/s11130-020-00816-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00816-0