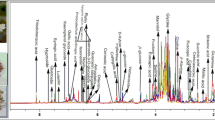
Abstract
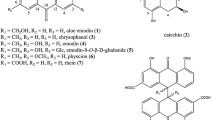
Previously, we established a 1H NMR metabolomics method using reversed-phase solid-phase extraction column (RP-SPEC), and succeeded in distinguishing wild from cultivated samples of Saposhnikoviae radix (SR), and between SR and its substitute, Peucedanum ledebourielloides root (PR). Herein, we performed LC-HR/MS metabolomics using fractions obtained via RP-SPEC to identify characteristic components of SR and PR. One and three characteristic components were respectively found for SR and PR; these components were isolated with their m/z values and retention times as a guide. The characteristic component of SR was identified as 4′-O-β-d-glucosyl-5-O-methylvisamminol (1), an indicator component used to identify SR in the Japanese Pharmacopoeia. In contrast, the characteristic components of PR were identified as xanthalin (2), 4′-O-β-d-apiosyl (1 → 6)-β-d-glucosyl-5-O-methylvisamminol (3), and 3′-O-β-d-apiosyl (1 → 6)-β-d-glucosylhamaudol (4) based on spectroscopic data such as 1D- and 2D-NMR, MS, and specific optical rotation. Among them, 4 is a novel compound. For the correlation between the NMR metabolomics results in the present and our previous report, only 1 and 2 were found to correlate with the chemical shifts, and the other compounds had no correlation. As the chemical shifts for compounds 1, 3, and 4 were similar to each other, especially for the aglycone moiety, they could not be distinguished because of the sensitivity and resolution of 1H NMR. Accordingly, combining NMR and LC/MS metabolomics with their different advantages is considered useful for metabolomics of natural products. The series of methods used in our reports could aid in quality evaluations of natural products and surveying of marker components.
Graphic abstract





Similar content being viewed by others
References
Tai J, Cheung S (2017) Anti-proliferative and antioxidant activities of Saposhnikovia divaricata. Oncol Rep 18:227–234
Liao H, Li Q, Liu R, Liu J, Bi K (2014) Fingerprint analysis and multi-ingredient determination using a single reference standard for Saposhnikoviae Radix. Anal Sci 30:1157–1163
Kong X, Liu C, Zhang C, Zhao J, Wang J, Wan H, Zhu H, Zhang P, Chen W, Xiao Y, Lin N (2013) The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model. J Ethnopharmacol 148:842–850
Kim HS, Choi G, Lee AY (2018) Ultra-performance convergence chromatography method for the determination of four chromones and quality control of Saposhnikovia divaricata (Turcz.) Schischk. J Sep Sci 41:1682–1690
Batsukh Z, Toume K, Javzan B, Kazuma K, Cai S, Hayashi S, Kawahara N, Maruyama T, Komatsu K (2020) Metabolomic profiling of Saposhnikoviae Radix from Mongolia by LC–IT–TOF–MS/MS and multivariate statistical analysis. J Nat Med 74(1):170–188
Wang J, Materia LZ (1989) Medica research of Saposhunikoviae radix (provisional translation). Zhongguo Zhong Yao Za Zhi 14:579–581
Baba K, Qing X, Taniguchi M, Kozawa M, Fujita E (1991) Studies on Chiniese Medicine Fang-Feng (III) Constituents of Shui-Fang-Feng. Shoyakugaku Zasshi 45(2):167–173
Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi Yamazaki M (2001) Analgesic Components of Saposhnikovia Root (Saposhnikovia divaricata). Chem Pharm Bull 49(2):154–160
Maruyama T, Ezaki M, Shiba M, Yamaji H, Yoshitomi T, Kawano N, Cheng SZX, Yokokura T, Yamamoto Y, Fuchino H, Sun H, Komatsu K, Kawahara N (2018) Botanical origin and chemical constituents of commercial SR and its related crude drugs available in Shaanxi and the surrounding regions. J Nat Med 72:267–273
Yoshitomi T, Wakana D, Uchiyama N, Tsujimoto T, Kawano N, Yokokura T, Yamamoto Y, Fuchino H, Hakamatsuka T, Komatsu K, Kawahara N, Maruyama T (2020) 1H NMR based metabolomic analysis coupled with reversed-phase solid-phase extraction for sample preparation of Saposhnikovia roots and related crude drugs. J Nat Med 74:65–75
Afendi F, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, Takahashi H, Altaf-Ul-Amin M, Darusman L, Saito K, Kanaya S (2012) KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol 53:e1–12
Sun A, Feng L, Liu R (2006) Preparative isolation and purification of prim-O-glucosyl-cinmifugin and 4'-O--D-glucosyl-5-O-methylvisamminol from Radix saposhnikoviae by high speed countercurrent chromatography. J Liq Chromatogr Rel Technol 29:751–759
The Ministry of Health, Labor and Welfare, Japan (2016) The Japanese pharmacopoeia seventeenth edition
The Ministry of Health, Labor and Welfare in Japan (2019) Supplement II to the Japanese Pharmacopoeia seventeenth edition. The MHLW Notification No. 49
Kwon Y, Kim H, Kim M, Chun W (2017) Acetylcholinesterase Inhibitors from Angelica polymorpha Stem. Nat Pro Sci 23(2):97–102
Kramer M, Mühleis A, Conrad J, Leitenberger M, Beifuss U, Carle R, Kammerer DR (2011) Quantification of polyacetylenes in apiaceous plants by high-performance liquid chromatography coupled with diode array detection. Z Naturforsch C 66(7–8):319–327
El-Houri R, Kotowska D, Christensen KB, Bhattacharya S, Oksbjerg N, Wolber G, Kristiansen K, Christensen LP (2015) Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct 6:2135–2144
Bruins AP (1994) Atmospheric-pressure-ionization mass spectrometry: II. Applications in pharmacy, biochemistry and general chemistry. Trends Anal Chem 13(2):81–90
Sasaki H, Taguchi H, Endo T, Yoshioka I (1982) The Constituents of Ledebouriella seseloides WOLFF. I. Structures of Three New Chromones. Chem Pharm Bull 30(10):3555–3562
Zheleva A, Soine TO, Bubeva-Ivanova L (1972) Natural Coumarins V: Isolation of Xanthalin and a New Pyranocoumarin, Peuarenine, from Peucedanum arenarium. W K J Pharm Sci 61(10):1643–1644
Tanaka T, Nakashima T, Ueda T, Tomii K, Kouno I (2007) Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull 55(6):899–901
Wang YH, Avula B, Fu X, Wang M, Khan IA (2012) Simultaneous determination of the absolute configuration of twelve monosaccharide enantiomers from natural products in a single injection by a UPLC-UV/MS method. Planta Med 78:834–837
Acknowledgements
This study was supported by a research grant from the Japan Agency for Medical Research and Development (the grant number: 17ak0101074j2001, 15ak0101017h0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshitomi, T., Wakana, D., Uchiyama, N. et al. Identifying the compounds that can distinguish between Saposhnikovia root and its substitute, Peucedanum ledebourielloides root, using LC-HR/MS metabolomics. J Nat Med 74, 550–560 (2020). https://doi.org/10.1007/s11418-020-01409-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01409-6