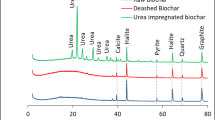
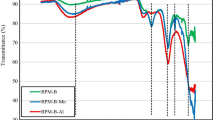
Abstract
The effectiveness of phosphate (P) removal from aqueous solutions was investigated by novel low-cost biochars synthesized from vinasse and functionalized with calcined dolomite. The vinasse-derived biochar, synthesized via pyrolysis at different temperatures, showed easy preparation and a large surface area. The novel vinasse biochar nanocomposites were prepared by adding dolomite to the vinasse biochars with different weight percentages (10, 20 and 30%). The characteristics of the prepared materials were identified for further understanding of the inherent adsorption mechanism between P ions and vinasse biochars. Vinasse-dolomite nanocomposite was very effective in the adsorption of P species from aqueous media. The effect of the operational factors on Vinasse-dolomite nanocomposite was explored by applying response surface methodology (RSM). According to RSM results, the optimum condition was achieved to be contact time 90 (min), 250 (mg/L) of P concentration and pH 7. Thermodynamic isotherm and kinetic studies were applied on experimental data to understand the adsorption behavior. The Vinasse-dolomite nanocomposite revealed preferential P species adsorption in the presence of co-existing anions. The P species could be recovered by 1.0 M HCl where the efficiency was not affected up to the fifth cycle. The P-loaded Vinasse-dolomite nanocomposite was successfully tested on a plant; it significantly improved its growth and proved its potency as a P-based fertilizer substitute.

Similar content being viewed by others
Change history
12 January 2021
Due to the typesetting error, there are some errors in the Equation (4) in the manuscript, the correction is as follows, and we apologize to the reader.
References
Aksu Z, Gönen F (2006). Binary biosorption of phenol and chromium (VI) onto immobilized activated sludge in a packed bed: Prediction of kinetic parameters and breakthrough curves. Separation and Purification Technology, 49(3): 205–216
Anbia M, Rahimi F (2017). Adsorption of platinum(IV) from an aqueous solution with magnetic cellulose functionalized with thiol and amine as a nano-active adsorbent. Journal of Applied Polymer Science, 134(39): 45361
Antunes E, Jacob M V, Brodie G, Schneider P A (2018). Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. Journal of Environmental Chemical Engineering, 6(1): 395–403
Aparicio J D, Benimeli C S, Almeida C A, Polti M A, Colin V L (2017). Integral use of sugarcane vinasse for biomass production of actinobacteria: Potential application in soil remediation. Chemosphere, 181: 478–484
Boeykens S, Piol M, Legal L, Saralegui A B, Vázquez C (2017). Eutrophication decrease: Phosphate adsorption processes in presence of nitrates. Journal of Environmental Management, 203
Chen M, Huo C, Li Y, Wang J (2016). Selective adsorption and efficient removal of phosphate from aqueous medium with graphenelanthanum composite. ACS Sustainable Chemistry & Engineering, 4(3): 1296–1302
Chen T, Zhang Y, Wang H, Lu W, Zhou Z, Zhang Y, Ren L (2014). Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresource Technology, 164: 47–54
Chowdhary P, Yadav A, Kaithwas G, Bharagava R N (2017). Green Technologies and Environmental Sustainability. Singh R and Kumar S, eds. Cham: Springer International Publishing, 409–435
Christofoletti C, Pedro Escher J, Correia J, Fernanda Urbano Marinho J, Silvia Fontanetti C (2013). Sugarcane vinasse: Environmental implications of its use. Waste Management, 33(12): 2752–2761
Creech J E, Monaco T A, Evans J O (2004). Photosynthetic and growth responses of zea mays L and four weed species following postemergence treatments with mesotrione and atrazinet. Pest Manag Sci, 60(11): 1079–1084
Deng L, Shi Z (2015). Synthesis and characterization of a novel Mg-Al hydrotalcite-loaded kaolin clay and its adsorption properties for phosphate in aqueous solution. Journal of Alloys and Compounds, 637: 188–196
Dubinin M, Radushkevich L V (1947). The equation of the characteristic curve of activated charcoal. Proceedings of the Union of Soviet Socialist Republics Academy of Sciences, 55: 331–337
Fang C, Zhang T, Li P, Jiang R, Wu S, Nie H, Wang Y (2015). Phosphorus recovery from biogas fermentation liquid by Ca-Mg loaded biochar. Journal of Environmental Sciences (China), 29: 106–114
Freundlich H (1907). Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie, 57(1): 385–470
Fukushima N A, Palacios-Bereche M C, Palacios-Bereche R, Nebra S A (2019). Energy analysis of the ethanol industry considering vinasse concentration and incineration. Renewable Energy, 142: 96–109
Hoarau J, Caro Y, Grondin I, Petit T (2018). Sugarcane vinasse processing: Toward a status shift from waste to valuable resource. A review. Journal of Water Process Engineering, 24: 11–25
Huang H, Liu J, Zhang P, Zhang D, Gao F (2017a). Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chemical Engineering Journal, 307: 696–706
Huang W, Zhang Y, Li D (2017b). Adsorptive removal of phosphate from water using mesoporous materials: A review. Journal of Environmental Management, 193: 470–482
Jung K W, Jeong T U, Choi J W, Ahn K H, Lee S H (2017). Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresource Technology, 244: 23–32
Jung K W, Jeong T U, Kang H J, Ahn K H (2016). Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresource Technology, 211: 108–116
Karimifard S, Alavi Moghaddam M R (2018). Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Science of the Total Environment, 640–641: 772–797
Kataki S, West H, Clarke M, Baruah D C (2016). Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resources, Conservation and Recycling, 107: 142–156
Kazak O, Ramazan Eker Y, Bingol H, Tor A (2017). Preparation of activated carbon from molasses-to-ethanol process waste vinasse and its performance as adsorbent material. Bioresource Technology, 241: 1077–1083
Kong L, Han M, Shih K, Su M, Diao Z, Long J, Chen D, Hou L A, Peng Y (2018). Nano-rod Ca-decorated sludge derived carbon for removal of phosphorus. Environmental Pollution, 233: 698–705
Langmuir I (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9): 1361–1403
Li R, Wang J J, Zhang Z, Awasthi M K, Du D, Dang P, Huang Q, Zhang Y, Wang L (2018a). Recovery of phosphate and dissolved organic matter from aqueous solution using a novel CaO-MgO hybrid carbon composite and its feasibility in phosphorus recycling. Science of the Total Environment, 642: 526–536
Li R, Wang J J, Zhou B, Awasthi M K, Ali A, Zhang Z, Gaston L A, Lahori A H, Mahar A (2016a). Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Science of the Total Environment, 559: 121–129
Li R, Wang J J, Zhou B, Awasthi M K, Ali A, Zhang Z, Lahori A H, Mahar A (2016b). Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresource Technology, 215: 209–214
Li Z, Qiu Z, Yang J, Ma B, Lu S, Qin C (2018b). Investigation of phosphate adsorption from an aqueous solution using spent fluid catalytic cracking catalyst containing lanthanum. Frontiers of Environmental Science & Engineering, 12(6): 15
López R, Antelo J, Fiol S, Macías-García F (2019). Phosphate adsorption on an industrial residue and subsequent use as an amendment for phosphorous deficient soils. Journal of Cleaner Production, 230: 844–853
Manning B A, Goldberg S (1996). Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Science Society of America Journal, 60(1): 121–131
Mitrogiannis D, Psychoyou M, Baziotis I, Inglezakis V J, Koukouzas N, Tsoukalas N, Palles D, Kamitsos E, Oikonomou G, Markou G (2017). Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite. Chemical Engineering Journal, 320: 510–522
Nugroho F L, Mulyatna L, Situmeang A D W (2014). Removal of phosphate from synthetic aqueous solution by adsorption with dolomite from Padalarang. Journal of Engineering and Technological Sciences, 46(4): 410–419
Pradel M, Aissani L (2019). Environmental impacts of phosphorus recovery from a “product” Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Science of the Total Environment, 656: 55–69
Ren S, Li M, Sun J, Bian Y, Zuo K, Zhang X, Liang P, Huang X (2017). A novel electrochemical reactor for nitrogen and phosphorus recovery from domestic wastewater. Frontiers of Environmental Science & Engineering, 11(4): 17
Rodrigues L A, Da Silva M L C P (2010). Thermodynamic and kinetic investigations of phosphate adsorption onto hydrous niobium oxide prepared by homogeneous solution method. Desalination, 263(1): 29–35
Salameh Y, Albadarin A B, Allen S, Walker G, Ahmad M (2015). Arsenic (III, V) adsorption onto charred dolomite: Charring optimization and batch studies. Chemical Engineering Journal, 259: 663–671
Selim A Q, Sellaoui L, Mobarak M (2019). Statistical physics modeling of phosphate adsorption onto chemically modified carbonaceous clay. Journal of Molecular Liquids, 279: 94–107
Shahid M K, Kim Y, Choi Y G (2019). Adsorption of phosphate on magnetite-enriched particles (MEP) separated from the mill scale. Frontiers of Environmental Science & Engineering, 13(5): 71
Sivakumar P, Palanisamy P (2009). Adsorption studies of basic Red 29 by a non-conventional activated carbon prepared from Euphorbia antiquorum L. International Journal of Chemtech Research, 1(3): 502–510
Stefaniak E, Biliński B, Dobrowolski R, Staszczuk P, Wojcik J (2002). The influence of preparation conditions on adsorption properties and porosity of dolomite-based sorbents. Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 208(1–3): 337–345
Sun G, Zhang C, Li W, Yuan L, He S, Wang L (2018). Effect of chemical dose on phosphorus removal and membrane fouling control in a UCT-MBR. Frontiers of Environmental Science & Engineering, 13(1): 1
Takaya C, Fletcher L, Singh S, Anyikude K, Ross A (2016a). Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere, 145: 518–527
Takaya C, Fletcher L, Singh S, Okwuosa U, Ross A (2016b). Recovery of phosphate with chemically modified biochars. Journal of Environmental Chemical Engineering, 4(1): 1156–1165
Tripathi M, Sahu J N, Ganesan P (2016). Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renewable & Sustainable Energy Reviews, 55: 467–481
Valle L R, Rodrigues S L, Ramos S J, Pereira H S, Amaral D C, Siqueira J O, Guilherme L R G (2016). Beneficial use of a by-product from the phosphate fertilizer industry in tropical soils: Effects on soil properties and maize and soybean growth. Journal of Cleaner Production, 112: 113–120
Vikrant K, Kim K H, Ok Y S, Tsang D C W, Tsang Y F, Giri B S, Singh R S (2018). Engineered/designer biochar for the removal of phosphate in water and wastewater. Science of the Total Environment, 616–617: 1242–1260
Wang J, Wang S (2019). Preparation, modification and environmental application of biochar: A review. Journal of Cleaner Production, 227: 1002–1022
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y, Wang L, Xiao H, Deng S (2015). Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere, 119: 646–653
Xue Y, Wang C, Hu Z, Zhou Y, Xiao Y, Wang T (2019). Pyrolysis of sewage sludge by electromagnetic induction: Biochar properties and application in adsorption removal of Pb(II), Cd(II) from aqueous solution. Waste Management (New York, N.Y.), 89: 48–56
Yao Y, Gao B, Chen J, Yang L (2013). Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environmental Science & Technology, 47(15): 8700–8708
Ye Y, Ngo H H, Guo W, Liu Y, Zhang X, Guo J, Ni B J, Chang S W, Nguyen D D (2016). Insight into biological phosphate recovery from sewage. Bioresource Technology, 218: 874–881
Zhao W, Shen A, Qiao Z, Pan L, Hu A, Zhang J (2018). Genetic types and distinguished characteristics of dolomite and the origin of dolomite reservoirs. Petroleum Exploration and Development, 45(6): 983–997
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Nanocomposites were prepared by adding dolomite to vinasse at different ratio.
• Textural and morphological features of adsorbents were studied in detail.
• CCD based RSM was used for investigation of P ion removal by nanocomposite.
• The qm based on Langmuir model for modified vinasse biochar was 178.57 mg/g.
• P loaded nanocomposite improved plant growth and could be utilized as P-fertilizer
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kamali, N., Mehrabadi, A.R., Mirabi, M. et al. Synthesis of vinasse-dolomite nanocomposite biochar via a novel developed functionalization method to recover phosphate as a potential fertilizer substitute. Front. Environ. Sci. Eng. 14, 70 (2020). https://doi.org/10.1007/s11783-020-1249-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-020-1249-6