Abstract
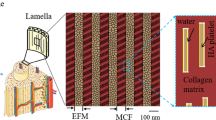
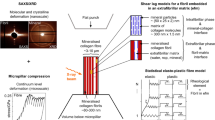
In this study, 3D finite element analyses (FEA) are conducted to quantify the orthotropic elastic properties and investigate the load transfer mechanism of bone at the sub-lamellar level. Three finite element (FE) unit cells with periodic boundary conditions are presented to model a two-scale microstructure of bone including a mineralized collagen fibril (MCF), the extrafibrillar matrix (EFM) and the resulting fibril array (FAY) under arbitrary loading. The axial and transverse elastic properties of the FAY computed by FEA are calibrated with unique experimental results on ovine micro-samples showing a coherent fibril orientation. They are then systematically compared with those calculated using analytical methods including the basic Voigt, Reuss and shear-lag models, the Mori–Tanaka scheme and the upper and lower bounds by Hashin and Shtrikman. The predicted axial strain ratios between the two-scales are discussed with respect to a recent small-angle X-ray scattering and wide-angle X-ray diffraction study. Beyond apparent elastic properties, the FE models provide stress distributions at both hierarchical levels, confirm the shear lag mechanisms within the MCF and between MCF and EFM and identify potential damage sites under arbitrary loading conditions. A comprehensive sensitivity analysis shows that mineral volume fraction in the fibril array is the dominant parameter on the axial and transverse elastic moduli, while the MCF volume fraction in FAY is the most sensitive variable for the ratio of axial versus transverse elastic modulus followed by the elastic moduli of hydroxyapatite and collagen. The FE model of the FAY developed and calibrated in the current study represents an anatomically realistic, experimentally validated and computationally efficient basis for investigating the apparent yield, post-yield and failure behaviors of lamellar bone in future research.










Similar content being viewed by others
References
Abueidda DW, Sabet FA, Jasiuk IM (2017) Modeling of stiffness and strength of bone at nano-scale. J Biomech Eng 139(5):051006
Adams J, Fantner GE, Fisher LW, Hansma PK (2008) Molecular energy dissipation in nano-scale networks of dentin matrix protein 1 is strongly dependent on ion valence. Nanotechnology 19(38):384008
Akiva U, Wagner HD, Weiner S (1998) Modelling the three-dimensional elastic constants of parallel-fibred and lamellar bone. J Mater Sci 33(6):1497–1509
Akkus O (2005) Elastic deformation of mineralized collagen fibrils: an equivalent inclusion based composite model. J Biomech Eng 127(3):383–390
Alexander B, Daulton TL, Genin GM, Lipner J, Pasteris JD, Wopenka B, Thomopoulos S (2012) The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen–mineral structure. J R Soc Interface 9(73):1774–1786
Al-Qtaitat AI, Aldalaen SM (2014) A review of non-Collagenous proteins; their role in bone. Am J Life Sci 2:351–355
Amaral M, Lopes MA, Silva RF, Santos JD (2002) Densification route and mechanical properties of Si3N4–bioglass biocomposites. Biomaterials 23(3):857–862
Aoubiza B, Crolet JM, Meunier A (1996) On the mechanical characterization of compact bone structure using the homogenization theory. J Biomech 29(12):1539–1547
Asgari M, Abi-Rafeh J, Hendy GN, Pasini D (2019) Material anisotropy and elasticity of cortical and trabecular bone in the adult mouse femur via AFM indentation. J Mech Behav Biomed Mater 93:81–92
Barkaoui A, Hambli R (2011) Finite element 3D modeling of mechanical behavior of mineralized collagen microfibrils. J Appl Biomater Biomech 9(3):199–206
Barkaoui A, Hambli R (2014) Nanomechanical properties of mineralised collagen microfibrils based on finite elements method: biomechanical role of cross-links. Comput Methods Biomech Biomed Eng 17(14):1590–1601
Barkaoui A, Chamekh A, Merzouki T, Hambli R, Mkaddem A (2014) Multi-scale approach including microfibril scale to assess elastic constants of cortical bone based on neural network computation and homogenization method. Int J Numer Methods Biomed Eng 30(3):318–338
Barkaoui A, Hambli R, Tavares JMR (2015) Effect of material and structural factors on fracture behaviour of mineralised collagen microfibril using finite element simulation. Comput Methods Biomech Biomed Eng 18(11):1181–1190
Barkaoui A, Tlili B, Vercher-Martínez A, Hambli R (2016) A multi-scale modelling of bone ultrastructure elastic proprieties using finite elements simulation and neural network method. Comput Methods Programs Biomed 134:69–78
Benveniste Y (1987) A new approach to the application of Mori–Tanaka's theory in composite materials. Mech Mater 6(2):147–157
Bhowmik R, Katti KS, Katti DR (2007) Mechanics of molecular collagen is influenced by hydroxyapatite in natural bone. J Mater Sci 42(21):8795–8803
Birk DE, Zycband EI, Woodruff S, Winkelmann DA, Trelstad RL (1997) Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev Dyn Off Publ Am Assoc Anat 208(3):291–298
Bishop N (2016) Bone material properties in osteogenesis imperfecta. J Bone Miner Res 31(4):699–708
Boskey AL (1992) Mineral–-matrix interactions in bone and cartilage. Clin Orthop Relat Res 281:244–274
Budiansky B (1965) On the elastic moduli of some heterogeneous materials. J Mech Phys Solids 13(4):223–227
Buehler MJ (2006) Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. J Mater Res 21(8):1947–1961
Buehler MJ (2007) Molecular nanomechanics of nascent bone: fibrillar toughening by mineralization. Nanotechnology 18(29):295102
Buehler MJ (2008) Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater 1(1):59–67
Buehler MJ, Keten S, Ackbarow T (2008) Theoretical and computational hierarchical nanomechanics of protein materials: deformation and fracture. Prog Mater Sci 53(8):1101–1241
Casari D, Pethö L, Schürch P, Maeder X, Philippe L, Michler J, Zysset P, Schwiedrzik J (2019a) A self-aligning microtensile setup: Application to single-crystal GaAs microscale tension–compression asymmetry. J Mater Res 34(14):2517–2534
Casari D, Michler J, Zysset P, Schwiedrzik J (2019b) A study of anisotropic strength tension–compression asymmetry in bone extracellular matrix. In: 25th congress of European society of biomechanics, July, 7–10, Vienna, Austria
Cassella JP, Stamp TCB, Ali SY (1996) A morphological and ultrastructural study of bone in osteogenesis imperfecta. Calcif Tissue Int 58(3):155–165
Cowin SC (2001) Bone mechanics handbook. CRC Press, Boca Raton
Cox HL (1952) The elasticity and strength of paper and other fibrous materials. Br J Appl Phys 3(3):72
Cribb AM, Scott JE (1995) Tendon response to tensile stress: an ultrastructural investigation of collagen: proteoglycan interactions in stressed tendon. J Anat 187(Pt 2):423
Currey JD (1969) The relationship between the stiffness and the mineral content of bone. J Biomech 2(4):477–480
Currey JD (2013) Bones: structure and mechanics. Princeton University Press, Princeton
Cusack S, Miller A (1979) Determination of the elastic constants of collagen by Brillouin light scattering. J Mol Biol 135(1):39–51
Deuerling JM, Yue W, Orías AAE, Roeder RK (2009) Specimen-specific multi-scale model for the anisotropic elastic constants of human cortical bone. J Biomech 42(13):2061–2067
Dong XN, Guo XE (2006) Prediction of cortical bone elastic constants by a two-level micromechanical model using a generalized self-consistent method. J Biomech Eng 128(3):309–316
Dubey DK, Tomar V (2008) Microstructure dependent dynamic fracture analyses of trabecular bone based on nascent bone atomistic simulations. Mech Res Commun 35(1–2):24–31
Eppell SJ, Tong W, Katz JL, Kuhn L, Glimcher MJ (2001) Shape and size of isolated bone mineralites measured using atomic force microscopy. J Orthop Res 19(6):1027–1034
Eppell SJ, Smith BN, Kahn H, Ballarini R (2005) Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3(6):117–121
Eshelby JD (1957) The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc R Soc Lond Ser A Math Phys Sci 241(1226):376–396
Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GA, Stucky GD, Morse DE, Hansma PK (2005) Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 4(8):612
Fantner GE, Adams J, Turner P, Thurner PJ, Fisher LW, Hansma PK (2007) Nano-scale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett 7(8):2491–2498
Franzoso G, Zysset PK (2009) Elastic anisotropy of human cortical bone secondary osteons measured by nanoindentation. J Biomech Eng 131(2):021001
Fratzl P (2003) Small-angle scattering in materials science-a short review of applications in alloys, ceramics and composite materials. J Appl Crystallogr 36(3):397–404
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52(8):1263–1334
Fratzl P, Groschner M, Vogl G, Plenk H Jr, Eschberger J, Fratzl-Zelman N, Koller K, Klaushofer K (1992) Mineral crystals in calcified tissues: a comparative study by SAXS. J Bone Miner Res 7(3):329–334
Fratzl P, Schreiber S, Boyde A (1996) Characterization of bone mineral crystals in horse radius by small-angle X-ray scattering. Calcif Tissue Int 58(5):341–346
Fratzl P, Gupta HS, Paschalis EP, Roschger P (2004) Structure and mechanical quality of the collagen–mineral nano-composite in bone. J Mater Chem 14(14):2115–2123
Fratzl-Zelman N, Schmidt I, Roschger P, Glorieux FH, Klaushofer K, Fratzl P, Rauch F, Wagermaier W (2014) Mineral particle size in children with osteogenesis imperfecta type I is not increased independently of specific collagen mutations. Bone 60:122–128
Fritsch A, Hellmich C (2007) Universal microstructural patterns in cortical and trabecular, extracellular and extravascular bone materials: micromechanics-based prediction of anisotropic elasticity. J Theor Biol 244(4):597–620
Gao H, Ji B, Jäger IL, Arzt E, Fratzl P (2003) Materials become insensitive to flaws at nano-scale: lessons from nature. Proc Natl Acad Sci 100(10):5597–5600
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2011) Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 11(2):757–766
Giraud-Guille MM (1988) Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif Tissue Int 42(3):167–180
Grant CA, Brockwell DJ, Radford SE, Thomson NH (2008) Effects of hydration on the mechanical response of individual collagen fibrils. Appl Phys Lett 92(23):233902
Groetsch A, Zysset PK, Pacareanu A, Peyrin F, Wolfram U (2019) An experimentally informed statistical model for the mechanical behaviour of mineralised collagen fibres. In: 25th congress of European society of biomechanics, July, 7–10, Vienna, Austria
Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P (2006) Cooperative deformation of mineral and collagen in bone at the nano-scale. Proc Natl Acad Sci 103(47):17741–17746
Halpin JC, Kardos JL (1976) The Halpin-Tsai equations: a review. Polym Eng Sci 16(5):344–352
Hamed E, Lee Y, Jasiuk I (2010) Multi-scale modeling of elastic properties of cortical bone. Acta Mech 213(1–2):131–154
Hamed E, Novitskaya E, Li J, Chen PY, Jasiuk I, McKittrick J (2012) Elastic moduli of untreated, demineralized and deproteinized cortical bone: validation of a theoretical model of bone as an interpenetrating composite material. Acta Biomater 8(3):1080–1092
Hang F, Barber AH (2010) Nano-mechanical properties of individual mineralized collagen fibrils from bone tissue. J R Soc Interface 8(57):500–505
Hang F, Gupta HS, Barber AH (2014) Nanointerfacial strength between non-collagenous protein and collagen fibrils in antler bone. J R Soc Interface 11(92):20130993
Hansma PK, Fantner GE, Kindt JH, Thurner PJ, Schitter G, Turner PJ, Udwin SF, Finch MM (2005) Sacrificial bonds in the interfibrillar matrix of bone. J Musculoskelet Neuronal Interact 5(4):313
Harley R, James D, Miller A, White JW (1977) Phonons and the elastic moduli of collagen and muscle. Nature 267(5608):285
Hashin Z, Shtrikman S (1963) A variational approach to the theory of the elastic behaviour of multiphase materials. J Mech Phys Solids 11(2):127–140
Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK (2004) High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35(1):4–10
He QC, Curnier A (1995) A more fundamental approach to damaged elastic stress-strain relations. Int J Solids Struct 32(10):1433–1457
Heim AJ, Matthews WG, Koob TJ (2006) Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl Phys Lett 89(18):181902
Hellmich C, Barthélémy JF, Dormieux L (2004) Mineral–collagen interactions in elasticity of bone ultrastructure–a continuum micromechanics approach. Eur J Mech A/Solids 23(5):783–810
Hershey AV (1954) The elasticity of an isotropic aggregate of anisotropic cubic crystals. J Appl Mech Trans ASME 21(3):236–240
Hibbitt K (2013) ABAQUS: user's manual: version 6.13
Hill R (1963) Elastic properties of reinforced solids: some theoretical principles. J Mech Phys Solids 11(5):357–372
Hirsch TJ (1962) Modulus of elasticity of concrete affected by elastic moduli of cement paste matrix and aggregate. In: Journal proceedings, vol 59, no 3, pp 427–452
Hofmann H, Voss T, Kühn K, Engel J (1984) Localization of flexible sites in thread-like molecules from electron micrographs: comparison of interstitial, basement membrane and intima collagens. J Mol Biol 172(3):325–343
Huang J, Wang X, Zhang TL, Wang K (2009) Alterations of ovariectomized rat bone and impact of non-collagenous proteins on mineralization. Joint Bone Spine 76(2):176–183
Ingram RT, Clarke BL, Fisher LW, Fitzpatrick LA (1993) Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Miner Res 8(9):1019–1029
Jackson SA, Cartwright AG, Lewis D (1978) The morphology of bone mineral crystals. Calcif Tissue Res 25(1):217–222
Jäger I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79(4):1737–1746
Jasiuk I, Ostoja-Starzewski M (2004) Modeling of bone at a single lamella level. Biomech Model Mechanobiol 3(2):67–74
Ji B, Gao H (2004) Mechanical properties of nanostructure of biological materials. J Mech Phys Solids 52(9):1963–1990
Kadler KE, Holmes DF, Trotter JA, Chapman JA (1996) Collagen fibril formation. Biochem J 316(1):1–11
Kasugai S, Todescan R Jr, Nagata T, Yao KL, Butler WT, Sodek J (1991) Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J Cell Physiol 147(1):111–120
Katz JL (1971) Hard tissue as a composite material—I. Bounds on the elastic behavior. J Biomech 4(5):455–473
Katz EP, Li ST (1973a) The intermolecular space of reconstituted collagen fibrils. J Mol Biol 73(3):351–369
Katz EP, Li ST (1973b) Structure and function of bone collagen fibrils. J Mol Biol 80(1):1–15
Katz JL, Ukraincik K (1971) On the anisotropic elastic properties of hydroxyapatite. J Biomech 4(3):221–227
Kröner E (1958) Berechnung der elastischen Konstanten des Vielkristalls aus den Konstanten des Einkristalls. Z Phys 151(4):504–518
Landis WJ (1996) Mineral characterization in calcifying tissues: atomic, molecular and macromolecular perspectives. Connect Tissue Res 34(4):239–246
Landis S, Price PA (1986) Disaggregation of bone into crystals. Calcif Tissue Int 39(6):365–375
Landis WJ, Silver FH (2002) The structure and function of normally mineralizing avian tendons. Comp Biochem Physiol A Mol Integr Physiol 133(4):1135–1157
Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF (1993) Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol 110(1):39–54
Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF (1996) Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech 33(2):192–202
Lees S (1987) Considerations regarding the structure of the mammalian mineralized osteoid from viewpoint of the generalized packing model. Connect Tissue Res 16(4):281–303
Lees S, Prostak KS, Ingle VK, Kjoller K (1994) The loci of mineral in turkey leg tendon as seen by atomic force microscope and electron microscopy. Calcif Tissue Int 55(3):180–189
Li S, Wang G (2008) Introduction to micromechanics and nanomechanics. World Scientific Publishing Company, Singapore
Lorenzo AC, Caffarena ER (2005) Elastic properties, Young's modulus determination and structural stability of the tropocollagen molecule: a computational study by steered molecular dynamics. J Biomech 38(7):1527–1533
Lusis J, Woodhams RT, Xanthos M (1973) The effect of flake aspect ratio on the flexural properties of mica reinforced plastics. Polym Eng Sci 13(2):139–145
Maghsoudi-Ganjeh M, Lin L, Wang X, Zeng X (2019) Computational investigation of ultrastructural behavior of bone using a cohesive finite element approach. Biomech Model Mechanobiol 18(2):463–478
Mann S, Webb JM, Williams RJP (eds) (1989) Biomineralization: chemical and biochemical perspectives. Wiley, Hoboken
Mbuyi-Muamba JM, Dequeker J, Gevers G (1989) Collagen and non-collagenous proteins in different mineralization stages of human femur. Cells Tissues Organs 134(4):265–268
McNally EA, Schwarcz HP, Botton GA, Arsenault AL (2012) A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS ONE 7(1):e29258
McNally E, Nan F, Botton GA, Schwarcz HP (2013) Scanning transmission electron microscopic tomography of cortical bone using Z-contrast imaging. Micron 49:46–53
Meng C, Heltsley W, Pollard DD (2012) Evaluation of the Eshelby solution for the ellipsoidal inclusion and heterogeneity. Comput Geosci 40:40–48
Miller A (1984) Collagen: the organic matrix of bone. Philos Trans R Soc Lond B Biol Sci 304(1121):455–477
Minary-Jolandan M, Yu MF (2009a) Nano-scale characterization of isolated individual type I collagen fibrils: polarization and piezoelectricity. Nanotechnology 20(8):085706
Minary-Jolandan M, Yu MF (2009b) Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromol 10(9):2565–2570
MJ S, BF M (1993) Mineral and organic matrix interaction in normally calcifying tendon visualized in 3 dimensions by high-voltage electron-microscopic tomography and graphic image-reconstruction. J Struct Biol 110(1):39–54
Morgan S, Poundarik AA, Vashishth D (2015) Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int 97(3):281–291
Mori T, Tanaka K (1973) Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall 21(5):571–574
Mortazavi B, Baniassadi M, Bardon J, Ahzi S (2013) Modeling of two-phase random composite materials by finite element, Mori-Tanaka and strong contrast methods. Compos B Eng 45(1):1117–1125
Mura T (2013) Micromechanics of defects in solids. Springer, Berlin
Nair AK, Gautieri A, Chang SW, Buehler MJ (2013) Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun 4:1724
Nanci A (1999) Content and distribution of noncollagenous matrix proteins in bone and cementum: relationship to speed of formation and collagen packing density. J Struct Biol 126(3):256–269
Nikel O, Laurencin D, McCallum SA, Gundberg CM, Vashishth D (2013) NMR investigation of the role of osteocalcin and osteopontin at the organic–inorganic interface in bone. Langmuir 29(45):13873–13882
Nikel O, Poundarik AA, Bailey S, Vashishth D (2018) Structural role of osteocalcin and osteopontin in energy dissipation in bone. J Biomech 80:45–52
Nikolov S, Raabe D (2008) Hierarchical modeling of the elastic properties of bone at submicron scales: the role of extrafibrillar mineralization. Biophys J 94(11):4220–4232
Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB (2007) Bone structure and formation: a new perspective. Mater Sci Eng R Rep 58(3–5):77–116
Orgel JP, Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ (2001) The in situ supermolecular structure of type I collagen. Structure 9(11):1061–1069
Padawer GE, Beecher N (1970) On the strength and stiffness of planar reinforced plastic resins. Polym Eng Sci 10(3):185–192
Paris O, Zizak I, Lichtenegger H, Roschger P, Klaushofer K, Fratzl P (2000) Analysis of the hierarchical structure of biological tissues by scanning X-ray scattering using a micro-beam. Cell Mol Biol (Noisy-le-Grand, France) 46(5):993–1004
Parnell WJ, Grimal Q (2008) The influence of mesoscale porosity on cortical bone anisotropy. Investigations via asymptotic homogenization. J R Soc Interface 6(30):97–109
Parry DA (1988) The molecular fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem 29(1–2):195–209
Paschalis EP, Gamsjaeger S, Fratzl-Zelman N, Roschger P, Masic A, Brozek W, Hassler N, Glorieux FH, Rauch F, Klaushofer K, Fratzl P (2016) Evidence for a role for nanoporosity and pyridinoline content in human mild osteogenesis imperfecta. J Bone Miner Res 31(5):1050–1059
Piekarski K (1973) Analysis of bone as a composite material. Int J Eng Sci 11(6):557–565
Poundarik A, Karim L, Gundberg C, Vashishth D (2009) The role of osteocalcin in bone fracture. In: Proceedings of the 55th meeting of the orthopaedic research society, pp 22–25
Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D (2012) Dilatational band formation in bone. Proc Natl Acad Sci 109(47):19178–19183
Prostak KS, Lees S (1996) Visualization of crystal-matrix structure. In situ demineralization of mineralized turkey leg tendon and bone. Calcif Tissue Int 59(6):474–479
Qiu SR, Wierzbicki A, Orme CA, Cody AM, Hoyer JR, Nancollas GH, Zepeda S, De Yoreo JJ (2004) Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc Natl Acad Sci 101(7):1811–1815
Raspanti M, Congiu T, Guizzardi S (2002) Structural aspects of the extracellular matrix of the tendon: an atomic force and scanning electron microscopy study. Arch Histol Cytol 65(1):37–43
Ravaglioli A, Krajewski A (1991) Bioceramics: materials·properties applications. Springer, Berlin
Reisinger AG, Pahr DH, Zysset PK (2010) Sensitivity analysis and parametric study of elastic properties of a unidirectional mineralized bone fibril-array using mean field methods. Biomech Model Mechanobiol 9(5):499–510
Reisinger AG, Pahr DH, Zysset PK (2011a) Principal stiffness orientation and degree of anisotropy of human osteons based on nanoindentation in three distinct planes. J Mech Behav Biomed Mater 4(8):2113–2127
Reisinger AG, Pahr DH, Zysset PK (2011b) Elastic anisotropy of bone lamellae as a function of fibril orientation pattern. Biomech Model Mechanobiol 10(1):67–77
Reuss A (1929) Berechnung der fließgrenze von mischkristallen auf grund der plastizitätsbedingung für einkristalle. ZAMM J Appl Math Mech/Z Angew Math Mech 9(1):49–58
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10(9):3815–3826
Reznikov N, Bilton M, Lari L, Stevens MM, Kröger R (2018) Fractal-like hierarchical organization of bone begins at the nanoscale. Science 360(6388):eaao2189
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20(2):92–102
Rice RV, Casassa EF, Kerwin RE, Maser MD (1964) On the length and molecular weight of tropocollagen from calf skin. Arch Biochem Biophys 105(2):409–423
Ritchie RO, Buehler MJ, Hansma P (2009) Plasticity and toughness in bone. Phys Today 62(6):41–47
Roach HI (1994) Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol Int 18(6):617–628
Robinson RA (1952) An electron-microscopic study of the crystalline inorganic component of bone and its relationship to the organic matrix. JBJS 34(2):389–476
Rubin MA, Jasiuk I, Taylor J, Rubin J, Ganey T, Apkarian RP (2003) TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 33(3):270–282
Sasaki N, Odajima S (1996) Stress–strain curve and Young's modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech 29(5):655–658
Sasaki N, Sudoh Y (1997) X-ray pole figure analysis of apatite crystals and collagen molecules in bone. Calcif Tissue Int 60(4):361–367
Schwarcz HP, McNally EA, Botton GA (2014) Dark-field transmission electron microscopy of cortical bone reveals details of extrafibrillar crystals. J Struct Biol 188(3):240–248
Schwarcz HP, Abueidda D, Jasiuk I (2017) The ultrastructure of bone and its relevance to mechanical properties. Front Phys 5:39
Schwiedrzik J, Raghavan R, Bürki A, LeNader V, Wolfram U, Michler J, Zysset P (2014) In situ micropillar compression reveals superior strength and ductility but an absence of damage in lamellar bone. Nat Mater 13(7):740
Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ (2010) In vitro fracture testing of submicron diameter collagen fibril specimens. Biophys J 99(6):1986–1995
Siegmund T, Allen MR, Burr DB (2008) Failure of mineralized collagen fibrils: modeling the role of collagen cross-linking. J Biomech 41(7):1427–1435
Silver FH, Freeman JW, Seehra GP (2003) Collagen self-assembly and the development of tendon mechanical properties. J Biomech 36(10):1529–1553
Spiesz EM, Zysset PK (2015) Structure–mechanics relationships in mineralized tendons. J Mech Behav Biomed Mater 1(52):72–84
Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10(2):141–150
Stevens MJ (2008) Simulation of the mechanical strength of a single collagen molecule. Biophys J 95(1):33–39
Tang Y, Ballarini R, Buehler MJ, Eppell SJ (2009) Deformation micromechanisms of collagen fibrils under uniaxial tension. J R Soc Interface 7(46):839–850
Tertuliano OA, Greer JR (2016) The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater 15(11):1195
Thurner PJ (2009) Atomic force microscopy and indentation force measurement of bone. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1(6):624–649
Thurner PJ, Chen CG, Ionova-Martin S, Sun L, Harman A, Porter A, Ager JW III, Ritchie RO, Alliston T (2010) Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 46(6):1564–1573
Tran VP, Brisard S, Guilleminot J, Sab K (2018) Mori-Tanaka estimates of the effective elastic properties of stress-gradient composites. Int J Solids Struct 146:55–68
Traub W, Arad T, Weiner S (1989) Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci 86(24):9822–9826
Urist MR, Strates BS (2009) The classic: bone morphogenetic protein. Clin Orthop Relat Res® 467(12):3051
Van Der Rijt JA, Van Der Werf KO, Bennink ML, Dijkstra PJ, Feijen J (2006) Micromechanical testing of individual collagen fibrils. Macromol Biosci 6(9):697–702
Vercher A, Giner E, Arango C, Tarancón JE, Fuenmayor FJ (2014) Homogenized stiffness matrices for mineralized collagen fibrils and lamellar bone using unit cell finite element models. Biomech Model Mechanobiol 13(2):437–449
Vercher-Martínez A, Giner E, Arango C, Fuenmayor FJ (2015) Influence of the mineral staggering on the elastic properties of the mineralized collagen fibril in lamellar bone. J Mech Behav Biomed Mater 42:243–256
Vesentini S, Fitié CF, Montevecchi FM, Redaelli A (2005) Molecular assessment of the elastic properties of collagen-like homotrimer sequences. Biomech Model Mechanobiol 3(4):224–234
Voigt W (1889) Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper. Ann Phys 274(12):573–587
Wagner HD, Weiner S (1992) On the relationship between the microstructure of bone and its mechanical stiffness. J Biomech 25(11):1311–1320
Wall P (1997) A comparison of homogenization, Hashin-Shtrikman bounds and the Halpin-Tsai equations. Appl Math 42(4):245–257
Wallace JM (2012) Applications of atomic force microscopy for the assessment of nano-scale morphological and mechanical properties of bone. Bone 50(1):420–427
Wang Y, Ural A (2018) Mineralized collagen fibril network spatial arrangement influences cortical bone fracture behavior. J Biomech 66:70–77
Weiner S, Traub W (1986) Organization of hydroxyapatite crystals within collagen fibrils. FEBS Lett 206(2):262–266
Weiner S, Traub W (1992) Bone structure: from angstroms to microns. FASEB J 6(3):879–885
Weiner S, Traub W, Wagner HD (1999) Lamellar bone: structure–function relations. J Struct Biol 126(3):241–255
Wenger MP, Bozec L, Horton MA, Mesquida P (2007) Mechanical properties of collagen fibrils. Biophys J 93(4):1255–1263
Wise ER, Maltsev S, Davies ME, Duer MJ, Jaeger C, Loveridge N, Murray RC, Reid DG (2007) The organic–mineral interface in bone is predominantly polysaccharide. Chem Mater 19(21):5055–5057
Withers PJ (1989) The determination of the elastic field of an ellipsoidal inclusion in a transversely isotropic medium, and its relevance to composite materials. Philos Mag A 59(4):759–781
Wu W, Owino J, Al-Ostaz A, Cai L (2014) Applying periodic boundary conditions in finite element analysis. In: SIMULIA community conference, Providence, pp 707–719
Yang L, van der Werf KO, Koopman BF, Subramaniam V, Bennink ML, Dijkstra PJ, Feijen J (2007) Micromechanical bending of single collagen fibrils using atomic force microscopy. J Biomed Mater Res Part A 82(1):160–168
Yang L, Van der Werf KO, Fitié CF, Bennink ML, Dijkstra PJ, Feijen J (2008) Mechanical properties of native and cross-linked type I collagen fibrils. Biophys J 94(6):2204–2211
Yao H, Ouyang L, Ching WY (2007) Ab initio calculation of elastic constants of ceramic crystals. J Am Ceram Soc 90(10):3194–3204
Yuan F, Stock SR, Haeffner DR, Almer JD, Dunand DC, Brinson LC (2011) A new model to simulate the elastic properties of mineralized collagen fibril. Biomech Model Mechanobiol 10(2):147–160
Zappone B, Thurner PJ, Adams J, Fantner GE, Hansma PK (2008) Effect of Ca2+ ions on the adhesion and mechanical properties of adsorbed layers of human osteopontin. Biophys J 95(6):2939–2950
Acknowledgements
Philippe Zysset acknowledges grant no 165510 of the Swiss National Science Foundation (SNSF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh, E., Dehestani, M. & Zysset, P. An efficient two-scale 3D FE model of the bone fibril array: comparison of anisotropic elastic properties with analytical methods and micro-sample testing. Biomech Model Mechanobiol 19, 2127–2147 (2020). https://doi.org/10.1007/s10237-020-01328-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01328-1