Abstract
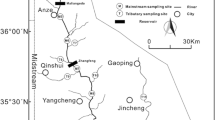
We present the application of dual stable isotope analyses of NO3 (δ15N-NO3 and δ18O-NO3) to provide a comprehensive assessment of the provenance, partitioning, and conversion of nitrate across the Day River Basin (DRB), Vietnam, which is heavily impacted by agriculture and urbanization. Stable isotope compositions of river water δ18O-H2O, in addition to their δ15N-NO3 and δ18O-NO3 signatures, were sampled at 12 locations in the DRB. Sample collection was conducted during three different periods to capture changes in regional weather and agricultural fertilization regimes; April (the dry season and key fertilization period), July (the rainy season and another key fertilization period) and October (the rainy season with no regional fertilization). Ranges of NO3 stable isotopes are − 7.1 to + 9.2‰ and − 3.9 to + 13.2‰ for δ18O and δ15N, respectively. Interpretation of the stable isotope data characterizes 4 main sources of NO3 in the DRB; (1) nitrified urea fertilizer derived from an intensive agricultural irrigation network, (2) soil and groundwater leaching from within the basin (3) manure and sewage inputs (which is more prevalent in downstream river sections) and (4) upstream inflow from the Red River which discharges into the Day River through the Dao River. We applied a mixing model for the DRB consisting of 4 variables, representing these 4 different sources. The partition calculation shows that during the fertilization and rainy period of July, more than 45% of river NO3 is derived from nitrified urea sources. During the other sampling periods (April and October), manure and sewage contribute more than 50% of river NO3 and are derived from the middle portion of the DRB, where the Day River receives domestic wastewater from the Vietnamese capital, Hanoi. Stable isotope data of O and N reveal that nitrification processes are more prevalent in the rainy season than in dry season and that this predominantly takes place in paddy field agricultural zones. In general, data demonstrate that nitrate loss in the DRB is due to denitrification which takes place in polluted stretches of the river and dominates in the dry season. This study highlights that (i) domestic waste should be treated prior to its discharge into the Day River and (ii) the need for better catchment agricultural fertilization practices as large portions of fertilizer currently discharge into the river, which greatly impacts regional water quality.






Similar content being viewed by others
References
Amberger A, Schmidt H-L (1987) Natürliche Isotopengehalte von Nitrat als Indikatoren für dessen Herkunft. Geochim Cosmochim Acta Vol 51:2699–2705
Baker MA, Vervier P (2004) Hydrological variability, organic matter supply and denitrification in the Garonne River ecosystem. Freshw Biol 49(2):181–190
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isot Environ Health Stud 43:237–247
Battaglin W et al (2001) Chemical and isotopic evidence of nitrogen transformation in the Mississippi River, 1997–98. In: Hydrological processes, vol 15, pp 1285–1300
Buchwald C, Casciotti KL (2010) Oxygen isotopic fractionation and exchange during bacterial nitrite oxidation. Limnol Oceanogr 55(3):1064–1074
Burns DA, Kendall C (2002) Analysis of δ15N and δ18O to differentiate NO3—sources in runoff at two watersheds in the Catskill Mountains of New York. Water Resour Res 38(5):9-1-9-11
Burns DA, Boyer EW, Elliott EM, Kendall C (2009) Sources and transformations of nitrate from streams draining varying land uses: evidence from dual isotope analysis. J Environ Qual 5(3):1149–1159 38 )
Casciotti K et al (2007) Oxygen isotopes in nitrite: analysis, calibration and equilibration. Anal Chem Vol 79:2427–2436
Casciotti K, McIlvin MR, Buchwald C (2010) Oxygen isotopic fractionation and exchange during bacterial ammonia oxidation. Limnol Oceanogr 55(3):753–762
Chen F, Jia G, Chen J (2009) Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, south China. Biogeochemistry 94:163–174
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard Methods for the Examination of Water and Wastewater, 20th Edition. s.l.:APHA American Public Health Association
Coplen TB, Wassenaar LI (2015) LIMS for Lasers 2015 for achieving long-term accuracy and precision of δ2H, δ17O, and δ18O of waters using laser absorption spectrometry. Rapid Commun Mass Spectrom 29:2122–2130
DARD-Hanoi (2009) Guidance of chemical fertilizers utilization in Chuong My district, Ha Noi capital (in Vietnamese), s.l.: Department of Agriculture and Rural Development—Hanoi
DARD-Namdinh (2011) Guidance of chemical fertilizers utilization in Vu Ban district, Nam Dinh province (in Vietnamese), s.l.: Department of Agriculture and Rural Development— Nam Dinh
Do TN, Nishida K (2014) A nitrogen cycle model in paddy fields to improve material flow analysis: the Day-Nhue River Basin case study. Nutr Cycl Agroecosyst 01(11):215–226 100
Do NT, Trinh DA, Nishida K (2014) Modification of uncertainty analysis in adapted material flow analysis: Case study of nitrogen flows in the Day-Nhue River Basin, Vietnam. Resources, Conservation & Recycling, Volume 88, pp. 67–75
Do TN, Tran BV, Trinh AD, Kei KN (2019) Quantification of nitrogen load in a regulated river system in Vietnam by material flow analysis. J Mater Cycles Waste Manag 21(4):974–998
Duc TA et al (2007) Experimental investigation and modelling approach of the impact of urban wastewater on a tropical river; a case study of the Nhue River, Hanoi, Viet Nam. J Hydrol 2:Volume 334, pp. 347–358
Galloway JN et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 01 9:153–226
Giap TV et al (2007) Study of isotopic technical application to estimate origin of nitrogen composition of groundwater in Hanoi Area. s.n, Viet Nam
GSO (2014) Report on the census of rural, agriculture and aquaculture, s.l.: Vietnam General Statistics Office
GSO (2016) Statistical Year Book of Ha Noi, Ha Nam, Nam Dinh, Ninh Binh, Hoa Binh province, s.l.: Vietnam General Statistics Office
Hanh PTM et al (2010) Anthropogenic influence on surface water quality of the Nhue and Day sub-river systems in Vietnam. Environ Geochem Health 01(6):227–236 32
Johnson LT, Royer TV, Edgerton JM, Leff LG (2012) Manipulation of the dissolved organic carbon pool in an agricultural stream: responses in microbial community structure, denitrification, and assimilatory nitrogen uptake. Ecosystems 15:1027–1038
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: McDONNELL JJ (ed) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: R. RobertMichener & K. Lajtha, eds. Stable Isotopes in Ecology and Environmental Science, 2nd Edition. Oxford: Wiley-Blackwell, p. 594
Kurosawa K et al (2004) Monitoring of inorganic nitrogen levels in the surface and ground water of the Red River Delta, Northern Vietnam. Commun Soil Sci Plant Anal 35:1645–1662
Kurosawa K et al (2006) Temporal and spatial variations of inorganic nitrogen levels in surface and groundwater around Hanoi, Vietnam. Commun Soil Sci Plant Anal 3:403–415
Lin J et al (2019) Seasonality of nitrate sources and isotopic composition in the Upper Illinois River. J Hydrol 568:849–861
Luu TNM et al (2010) Hydrological regime and water budget of the Red River Delta (Northern Vietnam). J Asian Earth Sci 37:219–228
Luu TNM et al (2012) N, P, Si budgets for the Red River Delta (northern Vietnam): how the delta affects river nutrient delivery to the sea. Biogeochemistry 107:241–259
MARD (2008) Guidelines of fertilizers application for rice, s.l. Ministry of Agriculture and Rural Development, Vietnam
McIlvin MR, Altabet MA (2005) Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem 77:5589–5595
Michalski G, Kolanowski M, Riha KM (2015) Oxygen and nitrogen isotopic composition of nitrate in commercial fertilizers, nitric acid, and reagent salts. Isot Environ Health Stud 51:382–391
MONRE (2006) State of Environment in Vietnam, s.1. Ministry of Natural Resources and Environment, Vietnam
Panno S, Hackley K, Kelly W, Hwang H (2006) Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. J Environ Qual 35(2):495–504
Popescu R et al (2015) Using stable isotopes in tracing contaminant sources in an industrial area: a case study on the hydrological basin of the Olt River, Romania. Sci Total Environ 533:17–23
Quay PD et al (1995) The 18O:16O of dissolved oxygen in rivers and lakes in the Amazon Basin: Determining the ratio of respiration to photosynthesis rates in freshwaters. Limnol Oceanogr 40:718–729
Quynh LTP et al (2005) Nutrient (N, P) budgets for the Red River basin (Vietnam and China). Glob Biogeochem Cycles
Saiki M, Do TN, Cao TTH, Nishida K (2019) Temporal variation of stable isotope values for dissolved nitrogen compounds in paddy water environment. Vietnam Atomic Energy Institute, Ha Long
Seitzinger S et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16(6):2064–2090
Ta TT et al (2016) Interpretation of anthropogenic impacts (agriculture and urbanization) on tropical deltaic river network through the spatio-temporal variation of stable (N, O) isotopes of NO–3. Isot Environ Health Stud 52:487–497
Thibodeau B, Hélie J-F, Lehmann M (2013) Variations of the nitrate isotopic composition in the St. Lawrence River caused by seasonal changes in atmospheric nitrogen inputs, 3ed edn, vol 115
Trinh AD, Meysman F, Rochelle-Newall E, Bonnet MP (2012) Quantification of sediment-water interactions in a polluted tropical river through biogeochemical modeling. Glob Biogeochem Cycles, 26
Trinh DA, Luu MTN, Le QTP (2017) Use of stable isotopes to understand run-off generation processes in the Red River Delta. Hydrol Process 31:3827–3843
Turner R, Rabalais N, Justic D, Dortch Q (2003) Global patterns of dissolved N, P and Si in large rivers. Biogeochemistry 64(3):297–317
Vrzel J et al (2016) Determination of the sources of nitrate and the microbiological sources of pollution in the Sava River Basin. Sci Total Environ 573:1460–1471
Wankel S, Kendall C, Francis C, Paytan A (2006) Nitrogen sources and cycling in the San Francisco Bay Estuary: A nitrate dual isotopic composition approach. Limnol Oceanogr 51(4):1654–1664
Wassenaar LI, Coplen TB, Aggarwal PK (2014) Approaches for achieving long-term accuracy and precision of δ18O and δ2H for waters analyzed using laser absorption spectrometers. Environ Sci Technol 48:1123–1131
Widory D et al (2013) Improving the management of nitrate pollution in water by the use of isotope monitoring: the δ15N, δ18O and δ11B triptych. Isot Environ Health Stud 49:29–47
Zarnetske JP, Haggerty R, Wondzell SM, Baker MA (2011) Labile dissolved organic carbon supply limits hyporheic denitrification. J Geophys Res 114:G4
Acknowledgements
This work was supported by the RCUK-NAFOSTED [Grant Numbers NE/P014577/1]; and the NAFOSTED [Grant Number 105.08-2014.26]. Stable isotope analysis was performed as part of the IAEA-CRP program ‘Isotopes to Study Nitrogen Pollution and Eutrophication of Rivers and Lakes—F32007’. This paper is written with a financial support from the Graduate School of Science and Technology, Vietnam Academy of Science and Technology (GUST.STS.ĐT2017-ST02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Charles T. Driscoll
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Calculation of δ18O-NO3 based on water oxygen and dissolved oxygen isotopes
Appendix: Calculation of δ18O-NO3 based on water oxygen and dissolved oxygen isotopes
Nitrification occurs as a two-step process whereby ammonia is first converted to nitrite and the produced nitrite is then converted to nitrate. During the bacterial nitrification process, the biogeochemical sources of oxygen atoms are dioxygen (O2) and water (H2O). O2 is incorporated during the oxidation of ammonia to hydroxylamine (NH2OH), while H2O is incorporated during the oxidation of both hydroxylamine to nitrite and nitrite to nitrate. While the ratio of 1:2 oxygen atoms from O2 and H2O implied by these observations is commonly used to interpret the oxygen isotopic content of nitrate derived from bacterial nitrification (Kendall 1998; Burns and Kendall 2002; Wankel et al. 2006), the utilization of this ratio involves the assumptions that exchange and fractionation of oxygen isotopes during nitrification are minimal. Recent works (e.g. Casciotti et al. 2007; Casciotti et al. 2010; Buchwald and Casciotti, 2010) have presented oxygen isotopic exchange and fractionation during nitrification. In general, during bacterial ammonia oxidation, the produced δ18O-NO2 is computed as:
In which χAOB, εk,O2,εk,H2O,1, εeq, are respectively the fraction of nitrite oxygen atoms that have equilibrated with H2O during ammonia oxidation, the kinetic isotope effect for O2 incorporation, the kinetic isotope effect for H2O incorporation by hydroxylamine oxidoreductase, and the equilibrium isotope effect for nitrite equilibration with H2O.
Then, during bacterial nitrite oxidation, δ18O-NO3 is estimated as (exchange of oxygen atoms between nitrite and water is minimal; Buchwald & Casciotti, 2010):
whereas εk,H2O,2 is the kinetic isotope effect for water incorporation by nitrite oxidoreductase.
Literature review has shown that χAOB, εk,O2+ εk,H2O,1, εeq, and εk,H2O,2 are respectively + 0.15 ± 0.1‰, + 26.3 ± 7.7‰, + 14‰, and + 15.5 ± 3.8‰ (Casciotti et al. 2007; Casciotti et al. 2010; Buchwald & Casciotti, 2010).
Rights and permissions
About this article
Cite this article
Luu, T.N.M., Do, T.N., Matiatos, I. et al. Stable isotopes as an effective tool for N nutrient source identification in a heavily urbanized and agriculturally intensive tropical lowland basin. Biogeochemistry 149, 17–35 (2020). https://doi.org/10.1007/s10533-020-00663-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00663-w