Abstract
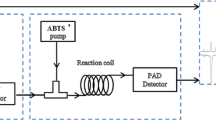
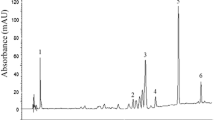
How to screen and evaluate the radical scavenging activity of each compound in the mixture remains a challenging project. In the present study, a sensitive and selective method of offline HPLC coupled with chemical methodology was developed for the evaluation of the radical scavenging activity of each compound in a common vegetable, crowndaisy (Chrysanthemum coronarium L.) seeds alcohol extract (CDAE) without the isolation of monomers. The results of LC–ESI–MS/MS showed that the main components of CDAE were chlorogenic acid (3-CQA) (1693.0 μg/g, dry weight), 1,3-dicaffeoylquinic acid (1,3-DCQA, 1378.5 μg/g), 3,5-DCQA (1027.0 μg/g), and 1,4-DCQA (1031.5 μg/g). The results of scavenging free radical activity analysis showed that, among all components, 1,3-DCQA, 3,5-DCQA, 1,4-DCQA, and 3-CQA were mainly responsible for the radical scavenging activity of the extract. Their scavenging rates for 2,2-diphenyl-1-picrylhydrazyl (DPPH) were 86.72%, 83.50%, 83.50%, and 83.43%, respectively. In the assay of scavenging the reactive oxygen species (ROS), CDAE could effectively decrease ROS in RAW264.7 cells without cell toxicity.





Similar content being viewed by others
References
Abdelgaleil SAM, Saad MMG, Ariefta NR, Shiono Y (2020) Antimicrobial and phytotoxic activities of secondary metabolites from Haplophyllum tuberculatum and Chrysanthemum coronarium. S Afr J Bot 128:35–41
Kang J, Chen LN, Zhao J, Sun XM, Zhang H (2014) The antitussive and expectorant effects of Tonghao extraction. Lishizhen Medicine and Materia Medica Research 25(1):8–9
Zhang JF, Yuan HL, Liu XB, Luan XS, Li CC (2012) Optimization of extraction of flavonoids from Chrysanthemum coronarium L. with response surface methodology. J Farm Mach 3:128–131
Ibrahim LF, El-Senousy WM, Hawas UW (2007) NMR spectral analysis of flavonoids from Chrysanthemum coronarium. Chem Nat Compd 43:659–662
Lin DY, You TT, Huang SY (2007) Extracting of total flavanone from Chrysanthemum coronarium herb and its effects on scavenging of hydroxyl radicals. Chin Wild Plant Resour 26(5):57–59
Chuda Y, Suzuki M, Nagata T, Tsushida T (1998) Contents and cooking loss of three quinic acid derivatives from garland (Chrysanthemum coronarium L.). J Agric Food Chem 46(4):1437–1439
Zhang LJ, Li R, Jiang ZT (2015) Extraction, purification and antioxidant activity of total flavonoids from Chrysanthemum coronarium L. leaves. Food Sci 36(24):40–45
Virág D, Király M, Drahos L, Édes AE, Gecse K, Bagdy G, Juhász G, Antal I, Klebovich I, Kiss BD, Ludányi K (2020) Development, validation and application of LC-MS/MS method for quantification of amino acids, kynurenine and serotonin in human plasma. J Pharm Biomed Anal 180:113018
Nie JY, Li R, Wang Y, Tan J, Tang SH, Jiang ZT (2019) Antioxidant activity evaluation of rosemary ethanol extract and their cellular antioxidant activity toward HeLa cells. J Food Biochem 43:e12851
Wang Y, Liu T, Li MF, Yang YS, Li R, Tan J, Tang SH, Jiang ZT (2019) Composition, cytotoxicity and antioxidant activities of polyphenols in the leaves of star anise (Illicium verum Hook. F.). ScienceAsia 45(6):532–537
Yang LC, Li R, Tan J, Jiang ZT (2013) Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J Agric Food Chem 61:1772–1778
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Wolfe KL, Kang XM, He XJ, Dong M, Zhang Q, Liu RH (2008) Cellular antioxidant activity of common fruits. J Agric Food Chem 56:8418–8426
Zhao Y, Wang Y, Jiang ZT, Li R (2017) Screening and evaluation of active compounds in polyphenol mixtures by HPLC coupled with chemical methodology and its application. Food Chem 227:187–193
Mullen W, Nemzer B, Ou B, Stalmach A, Hunter J, Clifford MN, Combet E (2011) The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J Agric Food Chem 59:3754–3762
Zhang WB, Wang ZC, Zhang LY (2013) Determination of 10 caffeoylquinic acids and 22 flavonoids in Chrysanthemum samples by ultra-high performance liquid chromatography-diode array detection tandem mass spectrometry. Chin J Anal Chem 41:1851–1861
Lin LZ, Harnly JM (2008) Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J Agric Food Chem 56:10105–10114
Lai JP, Lim YH, Su J, Shen HM, Ong CN (2007) Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J Chromatogr B 848:215–225
Gregoris E, Stevanato R (2010) Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem Toxicol 48:76–82
Kumazawa S, Hamasaka T, Nakayama T (2004) Antioxidant activity of propolis of various geographic origins. Food Chem 84:329–339
Yang H, Dong Y, Du H, Shi H, Peng Y, Li X (2011) Antioxidant compounds from propolis collected in Anhui, China. Molecules 16:3444–3455
Wan CP, Li SS, Liu L, Chen CY, Fan SY (2017) Caffeoylquinic acids from the aerial parts of Chrysanthemum coronarium L. Plants Basel 6:10
Acknowledgements
The present research was financially supported by the Natural Science Foundation of Tianjin City (Grant No. 17JCQNJC02400) and the National Training Programs for Innovation and Entrepreneurship of Undergraduates (Grant No. 201910859010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, R., Zhang, LJ., Jiang, ZT. et al. Radical scavenging activities and composition identification of phenolic compounds from crowndaisy seeds by offline HPLC combined with LC–ESI–MS/MS. Eur Food Res Technol 246, 1073–1080 (2020). https://doi.org/10.1007/s00217-020-03478-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03478-z