Abstract
Acute myeloid leukemia (AML) is a disease of the hematopoietic system that remains a therapeutic challenge despite advances in our understanding of the underlying cancer biology over the past decade. Recent developments in molecular targeting have shown promising results in treating leukemia, paving the way for novel treatment strategies. The discovery of drugs that promote apoptosis in leukemic cells has translated to encouraging activity in clinical trials. B-cell lymphoma (BCL)-2 inhibition has been at the center of drug development efforts to target apoptosis in AML. Remarkable clinical success with venetoclax has revolutionized the ways we treat hematological malignancies. Several landmark trials have demonstrated the potent antitumor activity of venetoclax, and it is now frequently combined with traditional cytotoxic agents to treat AML. However, resistance to BCL-2 inhibition is emerging, and alternative strategies to address resistance mechanisms have become an important focus of research. A number of clinical trials are now underway to investigate a plurality of novel agents that were shown to overcome resistance to BCL-2 inhibition in preclinical models. Some of the most promising data come from studies on drugs that downregulate myeloid cell leukemia (MCL)-1, such as cyclin-dependent kinases (CDK) inhibitors. Furthermore, innovative approaches to target apoptosis via extrinsic pathways and p53 regulation have added new cytotoxic agents to the arsenal, including drugs that inhibit inhibitor of apoptosis protein (IAP) family proteins and murine double minute 2 (MDM2). This review provides a perspective on past and current treatment strategies harnessing various mechanisms of apoptosis to target AML and highlights some important promising treatment combinations in development.

Similar content being viewed by others
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52. https://doi.org/10.1056/NEJMra1406184.
Kell J. Considerations and challenges for patients with refractory and relapsed acute myeloid leukaemia. Leuk Res. 2016;47:149–60. https://doi.org/10.1016/j.leukres.2016.05.025.
Luskin MR, Lee J-W, Fernandez HF, Abdel-Wahab O, Bennett JM, Ketterling RP, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551–8. https://doi.org/10.1182/blood-2015-07-657403.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–92. https://doi.org/10.1200/JCO.2017.77.6112.
von dem Borne PA, de Wreede LC, Halkes CJ, Marijt WA, Falkenburg JH, Veelken H. Effectivity of a strategy in elderly AML patients to reach allogeneic stem cell transplantation using intensive chemotherapy: long-term survival is dependent on complete remission after first induction therapy. Leuk Res. 2016;46:45–50. https://doi.org/10.1016/j.leukres.2016.03.010.
Bowman RL, Busque L, Levine RL. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell. 2018;22(2):157–70. https://doi.org/10.1016/j.stem.2018.01.011.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64. https://doi.org/10.1056/NEJMoa1614359.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–98. https://doi.org/10.1056/NEJMoa1716984.
Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–12. https://doi.org/10.1182/blood-2014-06-582809.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with %3e30% blasts. Blood. 2015;126(3):291–9. https://doi.org/10.1182/blood-2015-01-621664.
DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–28. https://doi.org/10.1016/s1470-2045(18)30010-x.
Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023–36. https://doi.org/10.1056/NEJMoa1605949.
Lubbert M, Ruter BH, Claus R, Schmoor C, Schmid M, Germing U, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97(3):393–401. https://doi.org/10.3324/haematol.2011.048231.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40. https://doi.org/10.1200/jco.2002.04.117.
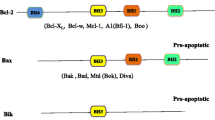
Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–96. https://doi.org/10.1016/j.coi.2007.05.004.
Leibowitz B, Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther. 2010;9(6):417–22. https://doi.org/10.4161/cbt.9.6.11392.
Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):147–71. https://doi.org/10.4161/cbt.4.2.1508.
Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein—a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197. https://doi.org/10.3389/fonc.2014.00197.
Mello SS, Attardi LD. Deciphering p53 signaling in tumor suppression. Curr Opin Cell Biol. 2018;51:65–72. https://doi.org/10.1016/j.ceb.2017.11.005.
Kastenhuber ER, Lowe SW. Putting p53 in context. Cell. 2017;170(6):1062–78. https://doi.org/10.1016/j.cell.2017.08.028.
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091–6.
Moon JH, Sohn SK, Lee M-H, Jang JH, Kim K, Jung CW, et al. BCL2 gene polymorphism could predict the treatment outcomes in acute myeloid leukemia patients. Leuk Res. 2010;34(2):166–72. https://doi.org/10.1016/j.leukres.2009.05.009.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–81. https://doi.org/10.1038/nature03579.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–8. https://doi.org/10.1158/0008-5472.Can-07-5836.
Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngelo DJ, et al. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS ONE. 2014;9(10):e108694. https://doi.org/10.1371/journal.pone.0108694.
Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18(11):3163–9. https://doi.org/10.1158/1078-0432.CCR-11-3090.
Bogenberger JM, Delman D, Hansen N, Valdez R, Fauble V, Mesa RA, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56(1):226–9. https://doi.org/10.3109/10428194.2014.910657.
Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernández-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338(9):564–71. https://doi.org/10.1056/NEJM199802263380902.
Zeuner A, Pedini F, Francescangeli F, Signore M, Girelli G, Tafuri A, et al. Activity of the BH3 mimetic ABT-737 on polycythemia vera erythroid precursor cells. Blood. 2009;113(7):1522–5. https://doi.org/10.1182/blood-2008-03-143321.
Hantel A, Wynne J, Lacayo N, Khaw SL, Rubnitz J, Mullighan C, et al. Safety and efficacy of the BCL inhibitors venetoclax and navitoclax in combination with chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:S184–S185185. https://doi.org/10.1016/j.clml.2018.07.016.
Lacayo NJ, Pullarkat VA, Stock W, Jabbour E, Bajel A, Rubnitz J, et al. Safety and efficacy of venetoclax in combination with navitoclax in adult and pediatric relapsed/refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Blood. 2019;134(Supplement_1):285. https://doi.org/10.1182/blood-2019-126977.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. https://doi.org/10.1038/nm.3048.
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–75. https://doi.org/10.1158/2159-8290.Cd-13-0609.
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–17. https://doi.org/10.1158/2159-8290.Cd-16-0313.
Tibes R BJ, Choudhary A, Monzon I, Chow D, Kiefer J, Azorsa O. RNA-based identification of novel sensitizers to 5-azacytidine in myeloid leukemias. In: Congress of the European Hematology Association, Berlin, Germany, June 4–7, 2009. Haematologica. 2009;94(supplement 2):1.
Bogenberger J, Shi C-X, Hagelstrom T, Gonzales I, Choudhary A, Tiedemann R, et al. Abstract LB-128: synthetic lethal RNAi screening identifies inhibition of Bcl-2 family members as sensitizers to 5-azacytidine in myeloid cells. Cancer Res. 2010;70(8 Supplement):LB-128. https://doi.org/10.1158/1538-7445.AM10-LB-128.
Bogenberger JM, Kornblau SM, Pierceall WE, Lena R, Chow D, Shi CX, et al. BCL-2 family proteins as 5-azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–65. https://doi.org/10.1038/leu.2014.44.
Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91(12):1861–70. https://doi.org/10.1007/s00277-012-1537-8.
Pollye DA, Dinardo CD, Thirman MJ, Letai A, Wei AH, Jonas BA, et al. Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy. J Clin Oncol. 2016;34(15_suppl):7009. https://doi.org/10.1200/JCO.2016.34.15_suppl.7009.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. https://doi.org/10.1182/blood-2018-08-868752.
Maurillo L, Venditti A, Spagnoli A, Gaidano G, Ferrero D, Oliva E, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer. 2012;118(4):1014–22. https://doi.org/10.1002/cncr.26354.
Bhatnagar B, Duong VH, Gourdin TS, Tidwell ML, Chen C, Ning Y, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014;55(7):1533–7. https://doi.org/10.3109/10428194.2013.856425.
Ritchie EK, Feldman EJ, Christos PJ, Rohan SD, Lagassa CB, Ippoliti C, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–7. https://doi.org/10.3109/10428194.2012.762093.
Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859–66. https://doi.org/10.1038/s41591-018-0233-1.
Lin TL, Strickland SA, Fiedler W, Walter RB, Hou J-Z, Roboz GJ, et al. Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia. J Clin Oncol. 2016;34(15_suppl):7007. https://doi.org/10.1200/JCO.2016.34.15_suppl.7007.
Wei AH, Strickland SA Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–84. https://doi.org/10.1200/jco.18.01600.
Maiti A, DiNardo CD, Cortes JE, Borthakur G, Pemmaraju N, Benton CB, et al. Interim analysis of phase II study of venetoclax with 10-day decitabine (DEC10-VEN) in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2018;132(Supplement 1):286. https://doi.org/10.1182/blood-2018-99-113749.
Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26(4):778–87. https://doi.org/10.1038/leu.2011.287.
Zhang Q, Han L, Shi C, Pan R, Ma MCJ, Ryan J, et al. Upregulation of MAPK/MCL-1 maintaining mitochondrial oxidative phosphorylation confers acquired resistance to BCL-2 inhibitor venetoclax in AML. Blood. 2016;128(22):101.
Daver N, Pollyea DA, Yee KWL, Fenaux P, Brandwein JM, Vey N, et al. Preliminary results from a phase Ib study evaluating BCL-2 inhibitor venetoclax in combination with MEK inhibitor cobimetinib or MDM2 inhibitor idasanutlin in patients with relapsed or refractory (R/R) AML. Blood. 2017;130(Suppl 1):813.
Juin P, Geneste O, Gautier F, Depil S, Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13(7):455–65. https://doi.org/10.1038/nrc3538.
Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91(3):991–1000.
Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114(24):5034–43. https://doi.org/10.1182/blood-2008-12-196055.
Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26(2):120–5. https://doi.org/10.1101/gad.182980.111.
Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM, et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep. 2016;6:27696. https://doi.org/10.1038/srep27696.
Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22(17):4440. https://doi.org/10.1158/1078-0432.CCR-15-3057.
Moujalled DM, Pomilio G, Ghiurau C, Ivey A, Salmon J, Rijal S, et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia. 2019;33(4):905–17. https://doi.org/10.1038/s41375-018-0261-3.
Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32(2):303–12. https://doi.org/10.1038/leu.2017.243.
Doi K, Liu Q, Gowda K, Barth BM, Claxton D, Amin S, et al. Maritoclax induces apoptosis in acute myeloid leukemia cells with elevated Mcl-1 expression. Cancer Biol Ther. 2014;15(8):1077–86. https://doi.org/10.4161/cbt.29186.
Caenepeel SR, Belmontes B, Sun J, Coxon A, Moody G, Hughes PE. Abstract 2027: preclinical evaluation of AMG 176, a novel, potent and selective Mcl-1 inhibitor with robust anti-tumor activity in Mcl-1 dependent cancer models. Cancer Res. 2017;77(13 Supplement):2027. https://doi.org/10.1158/1538-7445.AM2017-2027.
Hird AW, Secrist JP, Adam A, Belmonte MA, Gangl E, Gibbons F, et al. Abstract DDT01-02: AZD5991: a potent and selective macrocyclic inhibitor of Mcl-1 for treatment of hematologic cancers. Cancer Res. 2017;77(13 Supplement):DDT01–2. https://doi.org/10.1158/1538-7445.AM2017-DDT01-02.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–82. https://doi.org/10.1038/nature19830.
Papatzimas JW, Gorobets E, Maity R, Muniyat MI, MacCallum JL, Neri P, et al. From inhibition to degradation: targeting the antiapoptotic protein myeloid cell leukemia 1 (MCL1). J Med Chem. 2019;62(11):5522–40. https://doi.org/10.1021/acs.jmedchem.9b00455.
Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106(7):2513–9. https://doi.org/10.1182/blood-2005-04-1678.
Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8(11):3527–38.
Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96(2):393–7.
Lee DJ, Zeidner JF. Cyclin-dependent kinase (CDK) 9 and 4/6 inhibitors in acute myeloid leukemia (AML): a promising therapeutic approach. Expert Opin Investig Drugs. 2019;28(11):989–1001. https://doi.org/10.1080/13543784.2019.1678583.
Tibes R, Bogenberger JM. Transcriptional silencing of MCL-1 through cyclin-dependent kinase inhibition in acute myeloid leukemia. Front Oncol. 2019;9:1205.
Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–9. https://doi.org/10.3324/haematol.2015.125849.
Zeidner JF, Lin TL, Vigil CE, Dalovisio A, Wang ES, Levy MY, et al. Zella 201: a biomarker-guided phase II study of alvocidib followed by cytarabine and mitoxantrone in MCL-1 dependent relapsed/refractory acute myeloid leukemia (AML). Blood. 2018;132(Supplement 1):30. https://doi.org/10.1182/blood-2018-99-115018.
Bogenberger J, Whatcott C, Hansen N, Delman D, Shi CX, Kim W, et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget. 2017;8(63):107206–222. https://doi.org/10.18632/oncotarget.22284.
Kim W, Haws H, Peterson P, Whatcott CJ, Weitman S, Warner SL, et al. Abstract 5133: TP-1287, an oral prodrug of the cyclin-dependent kinase-9 inhibitor alvocidib. Cancer Res. 2017;77(13 Supplement):5133. https://doi.org/10.1158/1538-7445.AM2017-5133.
Gojo I, Sadowska M, Walker A, Feldman EJ, Iyer SP, Baer MR, et al. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol. 2013;72(4):897–908. https://doi.org/10.1007/s00280-013-2249-z.
Scholz A, Oellerich T, Hussain A, Lindner S, Luecking U, Walter AO, et al. Abstract 3022: BAY 1143572, a first-in-class, highly selective, potent and orally available inhibitor of PTEFb/CDK9 currently in phase I, shows convincing anti-tumor activity in preclinical models of acute myeloid leukemia (AML). Cancer Res. 2016;76(14 Supplement):3022. https://doi.org/10.1158/1538-7445.AM2016-3022.
Lucking U, Scholz A, Lienau P, Siemeister G, Kosemund D, Bohlmann R, et al. Identification of atuveciclib (BAY 1143572), the first highly selective, clinical PTEFb/CDK9 inhibitor for the treatment of cancer. ChemMedChem. 2017;12(21):1776–933. https://doi.org/10.1002/cmdc.201700447.
Olson CM, Jiang B, Erb MA, Liang Y, Doctor ZM, Zhang Z, et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2018;14(2):163–70. https://doi.org/10.1038/nchembio.2538.
Robb CM, Contreras JI, Kour S, Taylor MA, Abid M, Sonawane YA, et al. Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC). Chem Commun. 2017;53(54):7577–80. https://doi.org/10.1039/c7cc03879h.
Chen S, Dai Y, Pei XY, Myers J, Wang L, Kramer LB, et al. CDK inhibitors upregulate BH3-only proteins to sensitize human myeloma cells to BH3 mimetic therapies. Cancer Res. 2012;72(16):4225–377. https://doi.org/10.1158/0008-5472.Can-12-1118.
Höring E, Ott G, Bayha C, Markus K, Voehringer MC, van Der Kuip H, et al. Dual targeting of NOXA/MCL-1 synergistically induces cell death in mantle cell lymphoma (MCL) cells. Blood. 2015;126(23):2757. https://doi.org/10.1182/blood.V126.23.2757.2757.
Nguyen T, Parker R, Zhang Y, Hawkins E, Kmieciak M, Craun W, et al. Homoharringtonine interacts synergistically with bortezomib in NHL cells through MCL-1 and NOXA-dependent mechanisms. BMC Cancer. 2018;18(1):1129. https://doi.org/10.1186/s12885-018-5018-x.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388(6639):300–4. https://doi.org/10.1038/40901.
Schimmer AD, Pedersen IM, Kitada S, Eksioglu-Demiralp E, Minden MD, Pinto R, et al. Functional blocks in caspase activation pathways are common in leukemia and predict patient response to induction chemotherapy. Cancer Res. 2003;63(6):1242–8.
Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796–803.
Lacasse EC, Kandimalla ER, Winocour P, Sullivan T, Agrawal S, Gillard JW, et al. Application of XIAP antisense to cancer and other proliferative disorders: development of AEG35156/GEM640. Ann N Y Acad Sci. 2005;1058:215–34. https://doi.org/10.1196/annals.1359.032.
Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9(7):2826–36.
Shaw TJ, Lacasse EC, Durkin JP, Vanderhyden BC. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122(6):1430–4. https://doi.org/10.1002/ijc.23278.
Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells. Clin Cancer Res. 2003;9(7):2826.
Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27(28):4741–6. https://doi.org/10.1200/JCO.2009.21.8172.
Schimmer AD, Herr W, Hanel M, Borthakur G, Frankel A, Horst HA, et al. Addition of AEG35156 XIAP antisense oligonucleotide in reinduction chemotherapy does not improve remission rates in patients with primary refractory acute myeloid leukemia in a randomized phase II study. Clin Lymphoma Myeloma Leuk. 2011;11(5):433–8. https://doi.org/10.1016/j.clml.2011.03.033.
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11(2):519–27. https://doi.org/10.1016/s1097-2765(03)00054-6.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131(4):682–93. https://doi.org/10.1016/j.cell.2007.10.037.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131(4):669–81. https://doi.org/10.1016/j.cell.2007.10.030.
Carter BZ, Mak PY, Mak DH, Shi Y, Qiu Y, Bogenberger JM, et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014. https://doi.org/10.1093/jnci/djt440.
Frey NV, Luger S, Mangan J, Zebrowski A, Loren AW, Minderman H, et al. A phase I study using single agent birinapant in patients with relapsed myelodysplastic syndrome and acute myelogenous leukemia. Blood. 2014;124(21):3758. https://doi.org/10.1182/blood.V124.21.3758.3758.
Borthakur G, Foran JM, Wang ES, Rakkar A, Hager S, Frey NV, et al. A phase 1b/2a study of birinapant in combination with 5-azacitadine in patients with myelodysplastic syndrome who are naïve, refractory to or have relapsed on 5-azacitadine: a peliminary analysis. Blood. 2014;124(21):3263. https://doi.org/10.1182/blood.V124.21.3263.3263.
Borthakur G, Foran JM, Wang ES, Rakkar A, Minderman H, Burns J, et al. A phase 1b study of birinapant in combination with 5-azacitadine in patients with myelodysplastic syndrome who are naïve, refractory or have relapsed to 5-azacitadine. Blood. 2015;126(23):93. https://doi.org/10.1182/blood.V126.23.93.93.
Donnellan WB, Diez-Campelo M, Heuser M, Ritchie EK, Skolnik J, Font P, et al. A phase 2 study of azacitidine (5-AZA) with or without birinapant in subjects with higher risk myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML). J Clin Oncol. 2016;34(15_suppl):7060. https://doi.org/10.1200/JCO.2016.34.15_suppl.7060.
Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97(9):2784–90. https://doi.org/10.1182/blood.v97.9.2784.
Carter BZ, Wang RY, Schober WD, Milella M, Chism D, Andreeff M. Targeting survivin expression induces cell proliferation defect and subsequent cell death involving mitochondrial pathway in myeloid leukemic cells. Cell Cycle. 2003;2(5):488–93.
Erba HP, Sayar H, Juckett M, Lahn M, Andre V, Callies S, et al. Safety and pharmacokinetics of the antisense oligonucleotide (ASO) LY2181308 as a single-agent or in combination with idarubicin and cytarabine in patients with refractory or relapsed acute myeloid leukemia (AML). Investig New Drugs. 2013;31(4):1023–34. https://doi.org/10.1007/s10637-013-9935-x.
Khurana A, Shafer DA. MDM2 antagonists as a novel treatment option for acute myeloid leukemia: perspectives on the therapeutic potential of idasanutlin (RG7388). OncoTargets Ther. 2019;12:2903–10. https://doi.org/10.2147/OTT.S172315.
Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–23. https://doi.org/10.1038/nrc2012.
Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274(51):36031–4. https://doi.org/10.1074/jbc.274.51.36031.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–8. https://doi.org/10.1126/science.1092472.
Pishas KI, Al-Ejeh F, Zinonos I, Kumar R, Evdokiou A, Brown MP, et al. Nutlin-3a is a potential therapeutic for ewing sarcoma. Clin Cancer Res. 2011;17(3):494–504. https://doi.org/10.1158/1078-0432.Ccr-10-1587.
Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA. 2006;103(6):1888–933. https://doi.org/10.1073/pnas.0507493103.
Vu B, Wovkulich P, Pizzolato G, Lovey A, Ding Q, Jiang N, et al. Discovery of RG7112: a small-molecule MDM2 inhibitor in clinical development. ACS Med Chem Lett. 2013;4(5):466–9. https://doi.org/10.1021/ml4000657.
Long J, Parkin B, Ouillette P, Bixby D, Shedden K, Erba H, et al. Multiple distinct molecular mechanisms influence sensitivity and resistance to MDM2 inhibitors in adult acute myelogenous leukemia. Blood. 2010;116(1):71–80. https://doi.org/10.1182/blood-2010-01-261628.
Weisberg E, Halilovic E, Cooke VG, Nonami A, Ren T, Sanda T, et al. Inhibition of wild-type p53-expressing AML by the novel small molecule HDM2 inhibitor CGM097. Mol Cancer Ther. 2015;14(10):2249. https://doi.org/10.1158/1535-7163.MCT-15-0429.
Zhang W, Konopleva M, Burks JK, Dywer KC, Schober WD, Yang JY, et al. Blockade of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase and murine double minute synergistically induces apoptosis in acute myeloid leukemia via BH3-only proteins Puma and Bim. Cancer Res. 2010;70(6):2424–34. https://doi.org/10.1158/0008-5472.Can-09-0878.
Andreeff M, Kelly KR, Yee K, Assouline S, Strair R, Popplewell L, et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin Cancer Res. 2016;22(4):868. https://doi.org/10.1158/1078-0432.CCR-15-0481.
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56(14):5979–83. https://doi.org/10.1021/jm400487c.
Yee K, Martinelli G, Vey N, Dickinson MJ, Seiter K, Assouline S, et al. Phase 1/1b study of RG7388, a potent MDM2 antagonist, in acute myelogenous leukemia (AML) patients (Pts). Blood. 2014;124(21):116. https://doi.org/10.1182/blood.V124.21.116.116.
Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, et al. Synthetic lethality of combined Bcl-2 inhibition and p53 activation in AML: mechanisms and superior antileukemic efficacy. Cancer Cell. 2017;32(6):748–60.e6. https://doi.org/10.1016/j.ccell.2017.11.003.
Daver NG, Pollyea DA, Garcia JS, Jonas BA, Yee KWL, Fenaux P, et al. Safety, efficacy, pharmacokinetic (PK) and biomarker analyses of BCL2 inhibitor venetoclax (Ven) plus MDM2 inhibitor idasanutlin (idasa) in patients (pts) with relapsed or refractory (R/R) AML: a phase Ib, non-randomized, open-label study. Blood. 2018;132(Supplement 1):767. https://doi.org/10.1182/blood-2018-99-116013.
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/NEJMoa1513257.
Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392–400. https://doi.org/10.1182/blood-2017-06-788323.
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–33. https://doi.org/10.1200/jco.2016.70.4320.
Kaefer A, Yang J, Noertersheuser P, Mensing S, Humerickhouse R, Awni W, et al. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother Pharmacol. 2014;74(3):593–602. https://doi.org/10.1007/s00280-014-2530-9.
Tibes R, Kornblau S, Pierceall W, Lena R, Qiu YH, Cardone M, et al. BH3 profiling as predictor of 5-azacytidine and decitabine clinical responses. Blood. 2013;122(21):603. https://doi.org/10.1182/blood.V122.21.603.603.
Smith BD, Warner SL, Whatcott C, Siddiqui-Jain A, Bahr B, Dettman E, et al. An alvocidib-containing regimen is highly effective in AML patients through a mechanism dependent on MCL1 expression and function. J Clin Oncol. 2015;33(15_suppl):7062. https://doi.org/10.1200/jco.2015.33.15_suppl.7062.
Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8(339):339ra69. https://doi.org/10.1126/scitranslmed.aad3099.
Lalaoui N, Hanggi K, Brumatti G, Chau D, Nguyen NN, Vasilikos L, et al. Targeting p38 or MK2 enhances the anti-leukemic activity of smac-mimetics. Cancer Cell. 2016;30(3):499–500. https://doi.org/10.1016/j.ccell.2016.08.009.
Tibes R, McDonagh KT, Lekakis L, Bogenberger JM, Kim S, Frazer N, et al. Phase I study of the novel Cdc2/CDK1 and AKT inhibitor terameprocol in patients with advanced leukemias. Investig New Drugs. 2015;33(2):389–96. https://doi.org/10.1007/s10637-014-0198-y.
Ravandi F, Gojo I, Patnaik MM, Minden MD, Kantarjian H, Johnson-Levonas AO, et al. A phase I trial of the human double minute 2 inhibitor (MK-8242) in patients with refractory/recurrent acute myelogenous leukemia (AML). Leuk Res. 2016;48:92–100. https://doi.org/10.1016/j.leukres.2016.07.004.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Jun H. Choi has no conflicts of interest that might be relevant to the contents of this manuscript. Raoul Tibes and James M Bogenberger received writing support from Ashfield Healthcare Communications for an unrelated review article and have no conflicts of interest that are directly relevant to the content of this article. Raoul Tibes has received honorarium and travel support from Abbvie. James M Bogenberger has research funding pending from Tolero Pharmaceuticals for the preclinical investigation of alvocidib/TP-1287 in solid tumor models. Raoul Tibes and James M Bogenberger have published work on alvocidib in AML that is being used by pharmaceutical companies designing clinical trials; neither have received any personal or research compensation for this work.
Rights and permissions
About this article
Cite this article
Choi, J.H., Bogenberger, J.M. & Tibes, R. Targeting Apoptosis in Acute Myeloid Leukemia: Current Status and Future Directions of BCL-2 Inhibition with Venetoclax and Beyond. Targ Oncol 15, 147–162 (2020). https://doi.org/10.1007/s11523-020-00711-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00711-3