Abstract
Background
A taxane plus ramucirumab as second-line therapy followed by a checkpoint inhibitor (CPI) in third line has become a standard treatment strategy for advanced gastric cancer.
Objective
Herein, we investigated the impact of prior ramucirumab use on the efficacy of third-line immunotherapy and performed an exploratory analysis to identify potential biomarkers for the success of immunotherapy.
Patients and Methods
We retrospectively analyzed patients receiving CPI as a third-line treatment for advanced gastric cancer between January 2015 and March 2019. Clinicopathologic data, including patient characteristics, histopathologic reports, and treatment types and outcomes, were reviewed.
Results
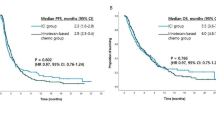
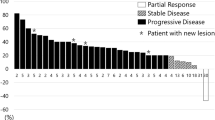
Of the 74 patients included in this study, 45 (61%) received nivolumab and 29 (39%) received pembrolizumab as a third-line CPI. For second-line therapy, 41 patients (55%) were treated with ramucirumab plus a taxane, and 33 (45%) received a chemotherapy regimen without ramucirumab. The disease control rates of CPIs were not statistically different according to prior use of ramucirumab. The overall survival (OS) with CPI was higher in patients receiving second-line therapy without ramucirumab compared with those receiving ramucirumab and taxane (5.6 vs 4.8 months, HR 0.56, 95% confidence interval [CI] 0.33–0.96; p = 0.03); however, this was not significant in a multivariate analysis. Patients achieving a response to second-line ramucirumab and a taxane showed greater benefit from subsequent CPI treatment compared with those not achieving a response (median OS 9.9 vs 2.3 months, HR 0.20, 95% CI 0.07–0.54; p < 0.001) as found in an exploratory analysis. Multivariate analysis also showed that prior response to ramucirumab and a taxane was an independent prognostic factor of OS with third-line CPI.
Conclusions
Response to ramucirumab and a taxane as a second-line treatment is an important prognostic marker for OS with subsequent third-line CPI. This data might provide useful information when applying CPIs as third-line therapies in advanced gastric cancer patients.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241(1):27–39.
Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241(2):247–55.
Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12(20):3237–42.
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013;24(11):2850–4.
Choi IS, Choi M, Lee JH, Kim JH, Suh KJ, Lee JY, et al. Treatment patterns and outcomes in patients with metastatic gastric cancer receiving third-line chemotherapy: a population-based outcomes study. PLoS One. 2018;13(6):e0198544.
Chan WL, Yuen KK, Siu SW, Lam KO, Kwong DL. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:68–81.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–26.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013.
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–8.
Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003–12.
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–7.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2). Gastr Cancer. 2019;22(2):344–54.
Komiya T, Huang CH, Neupane P, Williamson SK, Chalise P. Impact of previous anti-angiogenesis treatment in nivolumab-treated advanced non-small cell lung cancer. J Cancer Metastasis Treat. 2018;4:1.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–43.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–18.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of interest
Jinchul Kim, Seonggyu Byeon, Hyera Kim, Ja Hyun Yeo, Jung Yong Hong, Jeeyun Lee, Ho Yeong Lim, Won Ki Kang, and Seung Tae Kim declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, J., Byeon, S., Kim, H. et al. Impact of Prior Ramucirumab Use on Treatment Outcomes of Checkpoint Inhibitors in Advanced Gastric Cancer Patients. Targ Oncol 15, 203–209 (2020). https://doi.org/10.1007/s11523-020-00713-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00713-1