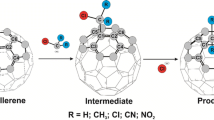
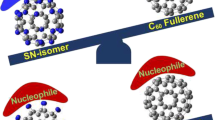
Abstract
In the current research, a three component reaction [reported experimentally by Li et al. (Org Lett 19:1192–1195, 2017)] between the C60 fullerene, indole and propargyl bromide is studied theoretically. According to the proposed mechanism in the experimental work, nucleophilic attack of indole anion (Ind−) to C60 affords fulleroindole monoanion (FI) as an intermediate, which experiences another nucleophilic attack on certain primary organohalides to generate the corresponding 1,4-(3-indole)(organo)[60]fullerenes. To investigate the reaction from the mechanistic point of view, certain competition paths were designed between the starting materials in each step and some theoretical quantities were obtained and elucidated. In consistence with the experimental findings, the local Fukui as well as Parr functions reactivity indices confirmed the observed regioselectivity in the first step of the reaction. These local reactivity indices implied also that in the nucleophilic attack of FI on the organohalides, one of the ortho positions is more nucleophilically activated than para position. A PES analysis on the proposed reaction paths revealed a good agreement with the experimental findings. The electron transfer studies and the computation of molecular electrostatic potential (MEP) map of the transition states suggested that in addition to the steric repulsions, the electronic repulsions in the ortho positions determines the para-selectivity preference over the ortho ones.







Similar content being viewed by others
References
Osawa E (1970) Superaromaticity. Chem Abstr 25:854–863
Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE (1985) C60: buckminsterfullerene. Nature 318:162–163
Hirsch A, Brettreich M (2005) Fullerenes: chemistry and reactions. WileyVCH, Weinheim
Wilson SR (2002) Nanomedicine: fullerene, carbon nanotube biology. Kluwer Academic, Dordrecht
Brabec CJ, Sariciftci NS, Hummelen JC (2001) Plastic solar cells. Adv Funct Mater 11:15–26
Bingel C (1993) Cyclopropanierung von Fullerenen. Chem Ber 126:1957–1959
Ivanova VN, Nadolonnyi VA, Grigoriev IA (2004) Synthesis and EPR investigation of nitroxyl derivatives of fullerenes C60 and C70. J Struct Chem 45:S71–S75
Wahl F, Weiler A, Landenberger P, Sackers E, Voss T, Haas A, Lieb M, Hunkler D, Wçrth J, Knothe L, Prinzbach H (2006) Towards perfunctionalized dodecahedranes-en route to C20 Fullerene. Chem Eur J 12:6255–6267
Nie B, Hasan K, Greaves MD, Rotello VM (1995) Reversible covalent attachment of C60 to a furan functionalized resin. Tetrahedron Lett 36:3617–3618
Schneider NS, Darwish AD, Kroto HW, Taylor R, Walton DRM (1994) Formation of fullerols via hydroboration of fullerene–C60. J Chem Soc Chem Commun 4:463–464
Soleymani M, Dashti Khavidaki H (2017) Inactivation possibility of pyrene by C20 fullerene via cycloaddition reactions: a theoretical study. Comput Theor Chem 1112:37–45
Soleymani M (2019) Theoretical study of the possibility of functionalization of C20 fullerene with the simplest ketene CH2CO. J Struct Chem 60:524–535
Siadati SA, Nami N (2016) Investigation of the possibility of functionalization of C20 fullerene by benzene via Diels-Alder reaction. Physica E 84:55–59
Anafcheh M, Ghafouri R (2014) Mono-and multiply-functionalized fullerene derivatives through 1, 3-dipolar cycloadditions: a DFT study. Physica E 56:351–356
Soleymani M, Dashti Khavidaki H (2020) Functionalization of the C20 fullerene by pyridine and pyrimidine: a theoretical study. Iran Chem Commun 8:111–122
Ikuma N, Nakagawa K, Kokubo K, Oshima T (2016) Regioselective addition of Grignard reagents to tosylazafulleroid and derivatization to 1,2-disubstituted [60]fullerene. Org Biomol Chem 14:7103–7108
Li CZ, Yip HL, Jen AKY (2012) Functional fullerenes for organic photovoltaics. J Mater Chem 22:4161–4177
Matsuo Y (2012) Design concept for high-LUMO-level fullerene electron-acceptors for organic solar cells. Chem Lett 41:754–759
Brabec CJ, Cravino A, Meissner D, Sariciftci NS, Fromherz T, Rispens MT, Sanchez L, Hummelen JC (2001) Origin of the open circuit voltage of plastic solar cells. Adv Funct Mater 11:374–380
Si W, Zhang X, Asao N, Yamamoto Y, Jin T (2015) Ni-Catalyzed direct 1,4-difunctionalization of [60]fullerene with benzyl bromides. Chem Commun 51:6392–6394
Lamparth I, Maichle-Mossmer C, Hirsch A (1995) Reversible template-directed activation of equatorial double bonds of the fullerene framework: regioselective direct synthesis, crystal structure, and aromatic properties of Th-C66(COOEt)12. Angew Chem Int Ed Engl 34:1607–1609
Caron C, Subramanian R, D’Souza F, Kim J, Kutner W, Jones MT, Kadish KM (1993) Selective electrosynthesis of dimethylfullerene [(CH3)2C60]: a novel method for the controlled functionalization of fullerenes. J Am Chem Soc 115:8505–8506
Keshavarz-K M, Knight B, Srdanov G, Wudl FJ (1995) Cyanodihydrofullerenes and dicyanodihydrofullerene: the first polar solid based on C60. J Am Chem Soc 117:11371–11376
Allard E, Riviere L, Delaunay J, Dubois D, Cousseau J (1999) Chemical generation and reactivity of C602−. High-yield and regioselective alkylation of [60, Ih] fullerene. Tetrahedron Lett 40:7223–7226
Subramanian R, Kadish KM, Vijayashree MN, Gao X, Jones MT, Miller MD, Krause KL, Suenobu T, Fukuzumi S (1996) Chemical generation of C602- and electron transfer mechanism for the reactions with alkyl bromides. J Phys Chem 100:16327–16335
Matsuo Y, Iwashita A, Abe Y, Li CZ, Matsuo K, Hashiguchi M, Nakamura E (2008) Regioselective synthesis of 1,4-di(organo)[60]fullerenes through DMF-assisted monoaddition of silylmethyl Grignard reagents and subsequent alkylation reaction. J Am Chem Soc 130:15429–15436
Matsuo Y, Sato Y, Niinomi T, Soga I, Tanaka H, Nakamura E (2009) Columnar structure in bulk heterojunction in solution-processable three-layered p-i-n organic photovoltaic devices using tetrabenzoporphyrin precursor and silylmethyl[60]fullerene. J Am Chem Soc 131:16048–16050
Zhang Y, Matsuo Y, Nakamura E (2011) Regiocontrolled synthesis of 1,2-di(organo)fullerenes via copper-assisted 1,4-aryl migration from silicon to carbon. Org Lett 13:6058–6061
Sundberg RJ (1970) The chemistry of indoles. Academic Press, New York
Humphrey GR, Kuethe JT (2006) Practical methodologies for the synthesis of indoles. Chem Rev 106:2875–2911
Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Verber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DH, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J (1988) Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem 31:2235–2246
Li F, Wang L, Wang J, Peng D, Zhao Y, Li S, Zhou H, Wu J, Tian X, Tian Y (2017) KOtBu-Mediated, three-component coupling reaction of indoles, [60]fullerene, and haloalkanes: one-pot, transition-metal-free synthesis of various 1,4-(3-indole)(organo)[60]fullerenes. Org Lett 19:1192–1195
Soleymani M, Kazemi Chegeni Z (2019) A molecular electron density theory study on the [3+2] cycloaddition reaction of 5,5-dimethyl-1-pyrroline N-oxide with 2-cyclopentenone. J Mol Graph Model 92:256–266
Soleymani M (2019) A density functional theory study on the [3 + 2] cycloaddition of N-(p-methylphenacyl)benzothiazolium ylide and 1-nitro-2-(p-methoxyphenyl) ethene: the formation of two diastereomeric adducts via two different mechanisms. Theor Chem Acc 138:87
Soleymani M, Jahanparvar S (2020) A computational study on the [3+2] cycloaddition of para-quinone methides with nitrile imines: a two-stage one-step mechanism. Mon Chem 151:51–61
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Zhao Y, Truhlar DG (2006) Comparative DFT study of van der Waals complexes: rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers. J Phys Chem 110:5121–5129
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Carpenter JE, Weinhold FJ (1988) Analysis of the geometry of the hydroxymethyl radical by the different hybrids for different spins' natural bond orbital procedure. J Mol Struct 169:41–62
Domingo LR, Perez P, Ortega DE (2013) Why do five-membered heterocyclic compounds sometimes not participate in polar Diels-Alder reactions? J Org Chem 78:2462–2471
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Yang W, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711
Domingo LR, Aurell MJ, Pêrez P, Contreras R (2002) Quantitative characterization of the local electrophilicity of organic molecules. Understanding the regioselectivity on Diels−Alder reactions. J Phys Chem A 106:6871–6875
Pêrez P, Domingo LR, Duque-Noreňa M, Chamorro E (2009) A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J Mol Struct 895:86–91
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 Revision E.01. Gaussian Inc., Wallingford
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874
Ess DH, Jones GO, Houk KN (2006) Conceptual, qualitative, and quantitative theories of 1,3-dipolar and Diels-Alder cycloadditions used in synthesis. Adv Synth Catal 348:2337–2361
Domingo LR, Aurell MJ, Perez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct 865:68–72
Laidler KJ (1941) Theories of Chemical Reaction Rates. McGraw-Hill, New York
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428
Acknowledgements
We are thankful to the Research Council and Office of Graduate Studies of the Ayatollah Boroujerdi University for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soleymani, M., Dashti Khavidaki, H. & Hosseini, M. Three-component coupling reaction of the C60 fullerene, indole and propargyl bromide: a theoretical study. Reac Kinet Mech Cat 130, 75–90 (2020). https://doi.org/10.1007/s11144-020-01776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01776-x