Abstract
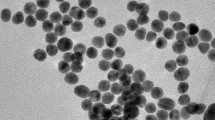
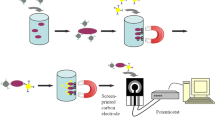
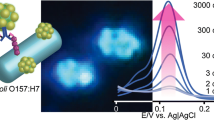
E. coli O157:H7 is one of the most important pathogens in food-borne diseases and is the main cause of the pseudo pandemic development of hemorrhagic colitis and hemolytic uremic syndrome. Also E. coli O157:H7 is the most common serotype of Shiga-toxin-producing E. coli. Traditional methods for detecting E. coli O157:H7 are expensive, time-consuming, and less sensitive. A method with high sensitivity and high-resolution optical detection is utilizes the LSPR property of spherical gold nanoparticles (GNP). In this work, we constructed a novel nano-bio probe to detect E. coli O157:H7 by synthesizing citrate gold nanoparticle conjugated (non-covalent bond) with specific chicken anti-E. coli O157:H7 antibody (IgY) by changing the pH of the nanoparticles’ environment. UV-visible and DLS methods were used to confirm the bonding between the antibody and nanoparticles and the LSPR sensitivity of the nano-bio probe was evaluated by ELISA method. We could optically detect this bacterium in less than 2 h by measuring the LSPR band λ max shifts of GNPs. The sensitivity of this novel biosensor was determined by about 10 CFU/ml, using the LSPR property of spherical gold nanoparticles. So that, the LSPR λ max red shifted from 530 to 543 nm in presence of 10 CFU bacterium. In conclusion, this nano biosensor can be used to detect this important pathogen among the clinical specimens.





Similar content being viewed by others
References
Karmali MA, Gannon V, Sargeant JM (2010) Verocytotoxin-producing Escherichia coli (VTEC). Vet Microbiol 140(3–4):360–370. https://doi.org/10.1016/j.vetmic.2009.04.011
Fairbrother JM, Nadeau E (2006) Escherichia coli: on-farm contamination of animals. Rev Sci Tech 25(2):555–569 http://www.ecl-lab.com/fr/news/documents/oierev08-fairbrother555-569.pdf. Accessed Sept 2006
Gyles C (2007) Shiga toxin-producing Escherichia coli: an overview. J Anim Sci 85(13):45–62 https://academic.oup.com/jas/article-abstract/85/suppl_13/E45/4775311. Accessed April 2007
Scheutz F (2014) Taxonomy meets public health: the case of Shiga toxin producing Escherichia coli. ASM Press, Washington
Yang X, Bai X, Zhang J, Sun H, Fu S, Fan R, et al (2019) Escherichia coli strains producing a novel Shiga toxin 2 subtype circulate in China. Int J Med Microbiol 151377. https://doi.org/10.1016/j.ijmm.2019.151377
Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA (2011) Shiga toxin subtypes display dramatic differences in potency. Infect Immun 79:1329–1337. https://doi.org/10.1128/IAI.01182-10
Ko H, Maymani H, Rojas-Hernandez C (2016) Hemolytic uremic syndrome associated with Escherichia coli O157:H7 infection in order adults: a case report and review of the literature. J Med Case Rep 10:17. https://doi.org/10.1186/s13256-016-0970
Razzaq S (2006) Hemolytic uremic syndrome: an emerging health risk. Am FAM Phys 74(6):991–996
Boyce TG, Swerdlow DL, Griffin PM (1995) Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med 333:364–368. https://doi.org/10.1056/NEJM199508103330608
Radke SM, Alocilja EC (2005) A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens Bioelectron 20(8):1662–1667. https://doi.org/10.1016/j.bios.2004.07.021
Piliarik M, Parova L, Homola J (2009) High-throughput SPR sensor for food safety. Biosens Bioelectron 24(5):1399–1404. https://doi.org/10.1016/j.bios.2008.08.012
Eum NS, Yeom SH, Kwon DH, Kim HR, Kang SW (2010) Enhancement of sensitivity using gold nanorods-antibody conjugator for detection of E. coli O157: H7, Sens. Actuators B: Chem 143:784–788. https://doi.org/10.1016/j.snb.2009.09.054
Homola J, Piliarik M (2006) Surface Plasmon resonance (SPR) sensors. Springer, Berlin
Green RJ, Frazier RA, Shakesheff KM, Davies MC, Roberts CJ, Tendler SJ (2000) Surface plasmon resonance analysis of dynamic biological interactions with biomaterials. Biomaterials 21(18):1823–1835. https://doi.org/10.1016/S0142-9612(00)00077-6
Kovacs-Nolan J, Mine Y (2004) Passive immunization through avian egg antibodies. Food Biotechnol 18:39e62. https://doi.org/10.1081/FBT-120030384
Barati B, Ebrahimi F, Nazarian S (2018) Production of chicken egg yolk antibody (IgY) against recombinant cholera toxin B subunit and evaluation of its prophylaxis potency in mice. Iran J Immunol 15(1):47–58
Sunwoo HH, Wang WW, Sim JS (2006) Detection of Escherichia coli O157:H7 using chicken immunoglobulin Y. Immunol Lett 106(2):191–193. https://doi.org/10.1016/j.imlet.2006.05.005
Wadkins RM, Golden JP, Pritsiolas LM, Ligler FS (1999) Detection of multiple toxic agents using a planar array immunosensor. Biosens Bioelectron 13(3–4):407–415. https://doi.org/10.1016/S0956-5663(97)00113-9
Lei JH, Guan F, Xu H, Chen L, Su BT, Zhou Y (2012) Application of an immunomagnetic bead ELISA based on IgY for detection of circulating antigen in urine of mice infected with Schistosoma japonicum. Vet Parasitol 187(1–2):196–202. https://doi.org/10.1016/j.vetpar.2011.12.017
Xiao Y, Gao X, Gannot E-BMR, Srivastava S, Wagner PD (2008) Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. Int J Cancer 122(10):2178–2186. https://doi.org/10.1002/ijc.23320
Bakhshi M, Ebrahimi F, Nazarian S, Zargan J, Behzadi F, Gariz DS (2017) Nano-encapsulation of chicken immunoglobulin (IgY) in sodium alginate nanoparticles: in vitro characterization. Biologicals. 49:69–75. https://doi.org/10.1016/j.biologicals.2017.06.002
Dong J, Carpinone PL, Pyrgiotakis G, Demokritou P, Moudgil BM (2019) Synthesis of precision gold nanoparticles using Turkevich method. Kona Powder Part J 1–8. https://doi.org/10.14356/kona.2020011
Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B (2016) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-Sens Res 9:17–22. https://doi.org/10.1016/j.sbsr.2016.04.002
Kootallur BN, Thangavelu CP, Mani M (2011) Bacterial identification in the diagnostic laboratory: how much is enough? Indian J Med Microbiol 94(4):336–340. https://doi.org/10.4103/0255-0857.90156
Houpikian P, Raoult D (2002) Traditional and molecular techniques for the study of emerging bacterial diseases: one laboratory's perspective. Emerg Infect Dis 8(2):122–131. https://doi.org/10.3201/eid0802.010141
Hammond JL, Bhalla N, Rafiee S, Estrela P (2014) Localized surface Plasmon resonance as a biosensing platform for developing countries. Biosensors 4:172–188. https://doi.org/10.3390/bios4020172
Zeinoddini M, Azizi A, Bayat S, Tavasoli Z (2018) Localized surface plasmon resonance (LSPR) detection of diphtheria toxoid using gold nanoparticle-monoclonal antibody conjugates. Plasmonics 13(2):583–590. https://doi.org/10.1007/s11468-017-0548-7
Huang X, El-Sayed MA (2010) Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res 1(1):13–28. https://doi.org/10.2217/17435889.2.5.681
Li B, Liu H, Wang W (2017) Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E.coli. BMC Microbiol 17:215. https://doi.org/10.1186/s12866-017-1123-2
Ibekwe AM, Watt PM, Grieve CM, Sharma VK, Lyons SR (2002) Multiplex fluorogenic real-time PCR for detection and quantification of Escherichia coli O157:H7 in dairy wastewater wetlands. App Environ Microbiol 68(10):4853–4862. https://doi.org/10.1128/AEM
Ranjbar R, Erfanmanesh M, Afshar D, Mohammadi M, Ghaderi O, Haghnazari A (2016) Visual detection of Enterohemorrhagic Escherichia coli O157:H7 using loop-mediated isothermal amplification. Electron Physician 8(6):2576–2585. https://doi.org/10.19082/2576
Pan TM, Chen LM, Su YC (2002) Identification of Escherichia coli O157:H7 by multiplex PCR with primers specific to the hlyA, eaeA, stx1, stx2fliC and rfb genes. J Formos Med Assoc 101(9):661–664 http://www.fma.org.tw/jfma/PDF/2002-101/Issue%2009/A10. Accessed Sept 2002
Csaki A, Stranik O, Fritzsche W (2018) Localized surface plasmon resonance based biosensing. Exp Rev Mol Diagnos 18(3):279–296. https://doi.org/10.1080/14737159.2018.1440208
Acknowledgments
We would like to thank the research council of The Malek-Ashtar University of Technology for the financial support of this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaghubi, F., Zeinoddini, M., Saeedinia, A.R. et al. Design of Localized Surface Plasmon Resonance (LSPR) Biosensor for Immunodiagnostic of E. coli O157:H7 Using Gold Nanoparticles Conjugated to the Chicken Antibody. Plasmonics 15, 1481–1487 (2020). https://doi.org/10.1007/s11468-020-01162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-020-01162-2