Abstract
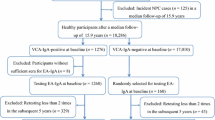
Epstein-Barr virus (EBV)-based serologic antibody testing has been found to be a feasible alternative for nasopharyngeal carcinoma (NPC) screening in endemic areas. The purpose of this study was to evaluate the performance of ELISA based on VCA IgA antibody, EA-IgA and Rta-IgG antibody specific to EBV in the diagnosis of NPC. A total of 2155 untreated NPC patients and 6957 healthy volunteers without nasopharyngeal disorder were recruited, and all subjects received EBV VCA-IgA, EA-IgA and Rta-IgG antibody tests simultaneously. The diagnostic efficiency of three testing alone or in combination for the diagnosis of NPC was evaluated. The prevalence of IgA antibody against EBV-VCA, IgA antibody against EBV-EA and IgG antibody against EBV-Rta was 89.9%, 46.6% and 63.2%. The sensitivity, specificity, positive predictive value, negative predictive value and Youden index were 89.88%, 89.65%, 73.18%, 96.63% and 0.79 for the EBV VCA-IgA antibody test, 46.59%, 96.89%, 82.5%, 85.42% and 0.43 for the EA-IgA antibody test, and 63.25%, 94.87%, 79.48%, 89.29% and 0.58 for the Rta-IgG antibody test in the diagnosis of NPC, and ROC curve analysis revealed the greatest diagnostic efficiency for EBV VCA-IgA antibody test and the lowest efficiency for EBV EA-IgA antibody test in the diagnosis of NPC. In addition, the simultaneous triple positivity of VCA-IgA, EA-IgA and Rta-IgG antibodies specific to EBV indicated the highest risk of NPC, and the simultaneous triple negativity of the three types of anti-EBV antibodies suggested the lowest risk of NPC. Our data demonstrate that EBV VCA-IgA antibody test shows a higher diagnostic efficiency than EA-IgA and Rta-IgG antibody tests for the screening of NPC, and triple positivity of is a better biomarker for the diagnosis of NPC.

Similar content being viewed by others
References
Chua MLK, Wee JTS, Hui EP, Chan ATC (2016) Nasopharyngeal carcinoma. Lancet 387:1012–1024
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS, Zeng YX, Jia WH (2016) Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 374:22–30
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Fu ZT, Guo XL, Zhang SW, Zeng HM, Sun KX, Chen WQ, He J (2018) Incidence and mortality of nasopharyngeal carcinoma in China, 2014. Zhonghua Zhong Liu Za Zhi 40:566–571
Petersson F (2015) Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 32:54–73
Kamran SC, Riaz N, Lee N (2015) Nasopharyngeal carcinoma. Surg Oncol Clin N Am 24:547–561
Tsao SW, Tsang CM, Lo KW (2017) Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci 372:20160270
Tsang CM, Tsao SW (2015) The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol Sin 30:107–121
Lee AW, Ma BB, Ng WT, Chan AT (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 33:3356–3364
Tabuchi K, Nakayama M, Nishimura B, Hayashi K, Hara A (2011) Early detection of nasopharyngeal carcinoma. Int J Otolaryngol. 2011:638058
Hildesheim A (2013) Epstein-Barr virus-based screening for the early detection of nasopharyngeal carcinoma: a new frontier. Am J Epidemiol 177:251–253
Ng MH, Chan KH, Ng SP, Zong YS (2006) Epstein-Barr virus serology in early detection and screening of nasopharyngeal carcinoma. Ai Zheng 25:250–256
Cao SM, Liu Z, Jia WH, Huang QH, Liu Q, Guo X, Huang TB, Ye W, Hong MH (2011) Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One 6:e19100
Lee AWM, Ng WT, Chan LK, Chan OSH, Hung WM, Chan CC, Cheng PTC, Sze H, Lam TS, Yau TK (2012) The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol 48:1007–1013
Chan AT, Grégoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, Felip E, EHNS–ESMO–ESTRO Guidelines Working Group (2012) Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii83–vii85
Gurtsevitch VE, Senyuta NB, Ignatova AV, Lomaya MV, Kondratova VN, Pavlovskaya AI, Dushenkina TE, Maximovich DM, Smirnova KV, Mudunov AM, Lichtenstein AV (2017) Epstein-Barr virus biomarkers for nasopharyngeal carcinoma in non-endemic regions. J Gen Virol 98:2118–2127
Anghel I, Anghel AG, Dumitru M, Soreanu CC (2012) Nasopharyngeal carcinoma -- analysis of risk factors and immunological markers. Chirurgia (Bucur) 107:640–645
Wong MM, Lye MS, Cheng HM, Sam CK (2005) Epstein-Barr virus serology in the diagnosis of nasopharyngeal carcinoma. Asian Pac J Allergy Immunol 23:65–67
Chen Y, Xin X, Cui Z, Zheng Y, Guo J, Chen Y, Lin Y, Su G (2016) Diagnostic value of serum Epstein-Barr virus capsid antigen-IgA for nasopharyngeal carcinoma: a meta-analysis based on 21 Studies. Clin Lab 62:1155–1166
Li Y, Wang K, Yin SK, Zheng HL, Min DL (2016) Expression of Epstein-Barr virus antibodies EA-IgG, Rta-IgG, and VCA-IgA in nasopharyngeal carcinoma and their use in a combined diagnostic assay. Genet Mol Res 15:gmr7368
Cai YL, Li J, Lu AY, Zheng YM, Zhong WM, Wang W, Gao JQ, Zeng H, Cheng JR, Tang MZ (2014) Diagnostic significance of combined detection of Epstein-Barr virus antibodies, VCA/IgA, EA/IgA, Rta/IgG and EBNA1/IgA for nasopharyngeal carcinoma. Asian Pac J Cancer Prev 15:2001–2006
Xia C, Zhu K, Zheng G (2015) Expression of EBV antibody EA-IgA, Rta-IgG and VCA-IgA and SA in serum and the implication of combined assay in nasopharyngeal carcinoma diagnosis. Int J Clin Exp Pathol 8:16104–16110
Sun P, Chen C, Cheng YK, Zeng ZJ, Chen XL, Liu LZ, Gu MF (2014) Serologic biomarkers of Epstein-Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 271:2545–2554
Sun R, Wang X, Li X (2015) Correlation Analysis of Nasopharyngeal Carcinoma TNM Staging with Serum EA IgA and VCA IgA in EBV and VEGF-C and -D. Med Sci Monit 21:2105–2109
Luo YL, Ou GP, Chi PD, Liang YN, Liu YH, Huang MY (2009) Combined determination of Epstein-Barr virus-related antibodies and antigens for diagnosis of nasopharyngeal carcinoma. Ai Zheng 28:76–78
Li S, Deng Y, Li X, Chen QP, Liao XC, Qin X (2010) Diagnostic value of Epstein-Barr virus capsid antigen-IgA in nasopharyngeal carcinoma: a meta-analysis. Chin Med J (Engl) 123:1201–1205
Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, Cheng G, Hu LF, Ernberg I, Zeng YX (2004) Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 100:1162–1170
Song C, Yang S (2013) A meta-analysis on the EBV DNA and VCA-IgA in diagnosis of Nasopharyngeal Carcinoma. Pak J Med Sci 29:885–890
Leung SF, Tam JS, Chan AT, Zee B, Chan LY, Huang DP, Van Hasselt A, Johnson PJ, Lo YM (2004) Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein-Barr virus DNA and anti-Epstein-Barr viral capsid antigen IgA antibody. Clin Chem 50:339–345
Chan KH, Gu YL, Ng F, Ng PS, Seto WH, Sham JS, Chua D, Wei W, Chen YL, Luk W, Zong YS, Ng MH (2003) EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer 105:706–709
Zhao FP, Liu X, Zhong ZM, Lu J, Yu BL, Zeng FY, Chen XM, Chen HH, Peng XH, Wang F, Peng Y, Li XP (2014) Positivity of both plasma Epstein-Barr virus DNA and serum Epstein-Barr virus capsid specific immunoglobulin A is a better prognostic biomarker for nasopharyngeal carcinoma. BBA Clin 2:88–93
Funding
This study was funded by the grants from the Natural Science Foundation of Fujian Province (grant nos. 2016J01511, 2017J05120 and 2018J01273), the Science and Technology Program of Fujian Province (grant no. 2018Y2003) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (grant no. 2017Y9072).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Cui, Z., Zheng, Y. et al. Comparison of Epstein-Barr Virus Serological Tools for the Screening and Risk Assessment of Nasopharyngeal Carcinoma: a Large Population-based Study. Pathol. Oncol. Res. 26, 2185–2190 (2020). https://doi.org/10.1007/s12253-020-00808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-020-00808-0