Highlights
-
A novel interface design of producing interfacial voids is proposed for CsPbIBr2 perovskite solar cells (PSCs), which is free of any extra modification layer.
-
Interfacial voids improve absorption of CsPbIBr2 film, reduce saturation current density, and enlarge built-in potential of the PSCs.
-
The PSC yields a superior efficiency of 10.20% with a record-high photovoltage of 1.338 V.
Abstract
A novel interface design is proposed for carbon-based, all-inorganic CsPbIBr2 perovskite solar cells (PSCs) by introducing interfacial voids between TiO2 electron transport layer and CsPbIBr2 absorber. Compared with the general interfacial engineering strategies, this design exempts any extra modification layer in final PSC. More importantly, the interfacial voids produced by thermal decomposition of 2-phenylethylammonium iodide trigger three beneficial effects. First, they promote the light scattering in CsPbIBr2 film and thereby boost absorption ability of the resulting CsPbIBr2 PSCs. Second, they suppress recombination of charge carriers and thus reduce dark saturation current density (J0) of the PSCs. Third, interfacial voids enlarge built-in potential (Vbi) of the PSCs, awarding increased driving force for dissociating photo-generated charge carriers. Consequently, the PSC yields the optimized efficiency of 10.20% coupled with an open-circuit voltage (Voc) of 1.338 V. The Voc achieved herein represents the best value among CsPbIBr2 PSCs reported earlier. Meanwhile, the non-encapsulated PSCs exhibit an excellent stability against light, thermal, and humidity stresses, since it remains ~ 97% or ~ 94% of its initial efficiency after being heated at 85 °C for 12 h or stored in ambient atmosphere with relative humidity of 30–40% for 60 days, respectively.

Similar content being viewed by others
1 Introduction
Organic–inorganic hybrid perovskite semiconductors have emerged as promising absorber materials for solar cells that are known as perovskite solar cells (PSCs), owing to their excellent optoelectronic properties, low cost, and versatile low-temperature processability of thin films [1,2,3,4]. Furthermore, the certified power conversion efficiency (PCE) of PSCs has reached up to 25.2% [5], approaching that of commercial crystalline silicon solar cells. However, the poor stability of organic–inorganic hybrid perovskite films under thermal, humidity, and light stresses brings a severe reliability issue to PSCs [3, 4, 6]. In addition, most of high-efficiency PSCs generally contain expensive and unstable organic hole transport layers (HTLs) coupled with noble metal electrodes. These functional films not only aggravate the instability problem but also burden the production cost of corresponding PSCs [3, 6, 7]. Hence, there is a growing attention to carbon-based, all-inorganic PSCs that avoid use of any HTLs and noble metal electrodes, in view of their multiply superiorities including upgraded stability, cost-effectiveness, and facile fabrication [7,8,9,10,11].
Currently, the absorber materials explored for carbon-based, all-inorganic PSCs largely include CsPbI3 [12, 13], CsPbI2Br [14,15,16], CsPbIBr2 [8, 17,18,19,20], and CsPbBr3 [7, 9, 21,22,23]. Among them, CsPbIBr2 holds the relatively well-balanced characters of bandgap and phase stability [8, 17,18,19, 24, 25]. Meanwhile, CsPbIBr2 is also applicable to build photovoltaic windows [26], semitransparent solar cells [27], water splitting systems [28], and tandem solar cells in conjunction with the state-of-the-art photovoltaic technologies [11]. Nevertheless, the PCEs of carbon-based, all-inorganic CsPbIBr2 PSCs are still far from their theoretical level [29, 30], and even lag behind that of the ones based on other analogs [13, 15, 23]. This dilemma can be largely attributed to their inferior open-circuit voltage (Voc) and fill factor (FF) values, which are eventually caused by the serious non-radiative recombination within CsPbIBr2 films and interfacial recombination in the PSCs.
To solve the above obstacles, great efforts have been dedicated in improving the quality of CsPbIBr2 films, targeting to suppress non-radiative recombination of charge carriers, by crystallization engineering or heteroatom replacement on Cs site or Pb site [19, 20, 25, 31]. For example, our previous work [19] demonstrated that modification of general one-step method with an intermolecular exchange reaction could produce a pinhole-free CsPbIBr2 film composed of micro-sized, [100] preferential grains, which finally enabled an optimized PCE of 9.16%. More recently, it was reported that Ba(II) doping could boost the grain sizes and crystallinity of CsPbIBr2 film, bringing out reduced trap states and suppressed non-radiative recombination. As a result, the PCE of corresponding PSC was boosted from 8.40 to 10.51% [31].
Interfacial modification with extra functional materials has been attempted to weaken interfacial recombination of charge carriers and therefore improve the PCEs of CsPbIBr2 PSCs [17, 18, 30, 32]. For example, the innovative modification of TiO2 electron transport layer (ETL) with CsBr or SmBr3 could strikingly improve the PSC’s PCE to over 10% coupled with desirable FF of ~ 0.70, which represents the highest PCE among CsPbIBr2 PSCs reported to date [18, 30]. Guo et al. [17] reported that surficial passivation of SnO2 ETL could enable the CsPbIBr2 PSCs with a record-high Voc of 1.31 V in spite of its low PCE of 2.06%. Clearly, these progresses verify the considerable feasibility of interfacial modification strategy in overcoming the obstacle faced by carbon-based, all-inorganic CsPbIBr2 PSCs. However, the extra interfacial modifiers inevitably complex the configuration of CsPbIBr2 PSCs and thus bring some new parasitic issues. For example, the modifiers multiply the interfaces of CsPbIBr2 PSCs, leading to an increased degradation risk [3, 33, 34]. Meanwhile, they will also alter the crystallization dynamics of atop CsPbIBr2 films [35, 36], which brings a new challenge to prepare high-quality CsPbIBr2 film. Thus, it is highly desired to develop more feasible interfacial engineering strategy to boost interfacial kinetics of charge carriers in carbon-based, all-inorganic CsPbIBr2 PSCs, thereby boosting its Voc and FF as well as PCE.
In this study, we propose an interfacial engineering strategy for carbon-based, all-inorganic CsPbIBr2 PSCs by making interfacial voids between TiO2 ETL and CsPbIBr2 film. This strategy avoids introducing any extra modification layer in ultimate PSCs compared with the conventional ones. Further, we reveal that the interfacial voids can stimulate enhanced absorption, decreased interfacial recombination of charge carriers, as well as enlarged built-in potential (Vbi) in resulting CsPbIBr2 PSCs. Hence, the optimized one yields the outstanding PCE of 10.20% coupled with a high Voc of 1.338 V, a FF of 0.65, and a short-circuit current density (Jsc) of 11.73 mA cm−2. The PCE is beyond nearly most of CsPbIBr2-based cells reported earlier, and particularly the Voc represents the highest level among them. Moreover, the PSC exhibits excellent photo-stability as well as long-term stability against thermal and humidity stresses.
2 Experimental Section
2.1 Materials and Reagents
Patterned FTO glass substrates (Pilkington, TEC-8, 8 Ω sq−1) were supplied by Yingkou OPV Tech New Energy Co., Ltd., China. Conductive carbon paste was bought from Shanghai MaterWin New Materials Co., Ltd., China. Lead bromide (PbBr2, ultra-dry, 99.999%), cesium iodide (CsI, ultra-dry, 99.998%), and dimethyl sulfoxide (DMSO, anhydrous, 99.8 +%), isopropanol (anhydrous, 99.5 +%) are received from Alfa-Assar. 2-phenylethylammonium iodide (PEAI, 99.99%) was purchased from Xi’an Polymer Light Technology Co., Ltd., China. All of them are used without further treatment or purification.
2.2 Preparation of PEAI-Loaded FTO/TiO2 Substrate
Firstly, patterned FTO glass with size 2 × 2.5 cm2 was washed with detergent, ultrapure water, acetone, and ethanol in an ultrasonic cleaner successively. Next, TiO2 sol was spin coated onto the FTO glass at 3500 rpm for 30 s. After being annealed at 500 °C for 60 min in ambient air, the FTO/TiO2 substrate was achieved. Finally, 100 μL 3 mg mL−1 PEAI in isometric deionized water and isopropanol was spin-coated on the substrate at 3500 rpm for 60 s and baked at 80 °C for 5 min in ambient air in order to load PEAI species onto FTO/TiO2 substrate.
2.3 Deposition of CsPbIBr2 Film
CsPbIBr2 precursor (1.0 M) was firstly prepared by dissolving 370.0 mg PbBr2 and 260.0 mg CsI in 1 mL DMSO at room temperature with vigorously stirring. Then, the PEAI-loaded FTO/TiO2 substrate was transferred into a N2-filled glovebox. And, 70 μL CsPbIBr2 precursor was spin-coated the substrate at 1500 rpm for 20 s and 5000 rpm for 60 s. After the spin-coating process, the sample was annealed at 280 °C for 15 min to form the crystallized CsPbIBr2 film. For comparison, a CsPbIBr2 film on FTO/TiO2 substrate without loading of PEAI species was also prepared according to the same procedures above.
2.4 Fabrication of Carbon-Based, All-Inorganic PSC
Carbon paste with the area of 0.10 cm2 was firstly coated on CsPbIBr2 film by screen-printing method. Then, the sample was heated at 120 °C for 15 min to promote evaporation of solvent of carbon paste. After cooling down naturally to room temperature, the carbon-based, all-inorganic PSC was achieved for further characterizations.
3 Results and Discussion
3.1 Formation Mechanism of CsPbIBr2 Films with Interfacial Voids
The main preparation procedures of CsPbIBr2 film with interfacial voids are shown in Scheme 1. To be brief, FTO/TiO2 substrate was firstly prepared according to the recipe described in our previous work [18, 19]. Then, 2-phenylethylammonium iodide (PEAI, C6H5CH2CH2NH3I) in isometric deionized water and isopropanol was deposited on FTO/TiO2 substrate by spin-coating method, with the aim of loading PEAI species on TiO2 surface. The successful loading of PEAI can be verified by the scanning electron microscope (SEM) results in Fig. S1. Next, CsPbIBr2 precursor was spin-coated onto the substrate by one-step spin-coating method. Finally, the sample was annealed at 280 °C for 15 min to promote the crystallization of CsPbIBr2 grains. It is noted that the release of PEAI species happened simultaneously during this high-temperature annealing stage, as discussed below.
We conducted X-ray photoelectron spectroscopy (XPS) measurement to examine the constituents of obtained CsPbIBr2 film. For comparison, a same CsPbIBr2 film that was prepared at a low annealing temperature of 100 °C was also investigated. The reason for adopting this low annealing temperature to prepare the control sample is that it cannot cause the sublimation or decomposition of PEAI molecules, as demonstrated previously [37, 38]. Figure 1a manifests that clear XPS peaks corresponding to Cs, Pb, I, and Br elements appear for the films annealed at either 280 or 100 °C, indicating that they are composed of CsPbIBr2 materials. However, no any signal corresponding to PEAI species can be measured from them, even though the FTO/TiO2 substrate contains PEAI species. The core-level N 1s XPS spectra in Fig. 1b further verify this fact, since the typical N 1s peak of PEAI species is absent [38].
In general, XPS measurement can only reflect surficial composition information of samples. To insight bulk composition of the concerned CsPbIBr2 films, they were redissolved in situ by N,N-dimethylformamide (DMF) solvent and annealed again at 90 °C for 10 min to evaporate the solvent away. As shown in Fig. 1a, after such treatment the additional Sn 3d peaks that originate from FTO/TiO2 substrate can be detected from both films, indicating the full dissolution of CsPbIBr2 films by DMF solvent and therefore the possible non-uniform composition distribution in the films or their interfaces is eliminated. In this manner, XPS measurement can be used to study bulk composition of CsPbIBr2 films reasonably. Once again, as exhibited in Fig. 1b, the N 1s peak located at 401.8 eV can be detected from CsPbIBr2 film prepared at 100 °C, manifesting the existence of PEAI species in it [37, 38]. By contrast, the peak is still missing in CsPbIBr2 film prepared at 280 °C, indicating that there is no detectable PEAI species in it indeed. This can be also supported by the energy-dispersive spectrometer (EDS) result in Fig. S2. We attribute such phenomenon to the sublimation of PEAI species during the high-temperature annealing stage of CsPbIBr2 film.
To support our standpoint, thermal property of PEAI powder was investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) [39, 40]. The TGA curve in Fig. 1c indicates that PEAI powder undergoes 100% weight loss in one step with the onset temperature of ~ 185 °C, suggesting that it is sublimated without complex decomposition procedure. This can be supported by the sharp transition step in corresponding differential TGA curve in Fig. S3. Moreover, the endothermic peak at ~ 283 °C in the DSC curve in Fig. 1d also suggests that the release of PEAI is related to a sublimation process. Thus, the typical sublimation temperature of PEAI powder is estimated to be ~ 283 °C, which is nearly the same as the annealing temperature of CsPbIBr2 film adopted in this work. Additionally, it is noted that the DSC measurement was performed on PEAI powder. In the case of within CsPbIBr2 thin film, a much larger surface-to-volume ratio is provided; therefore, the sublimation temperature could be lowered considerably to below ~ 283 °C for PEAI. Thus far, all the above observations provide conclusive evidences that in terms of CsPbIBr2 film prepared at 280 °C the original PEAI species on FTO/TiO2 substrate do not reside in its bulk or at its interface, but are sublimated during the thermal annealing stage.
3.2 Morphology Characterization
With this fact in mind, we turned to study the microstructure of CsPbIBr2 films deposited on FTO/TiO2 substrates without and with loading of PEAI species. Their top-view SEM images are provided in Fig. 2a, b. Clearly, both films are composed of closely packed crystalline grains without any discernible pinholes. Their grains are featured by dominant triple-junctions grain boundaries with equal vertex angle of 120°, implying that they are in equilibrium state with low internal stress [41, 42]. By contrast, the CsPbIBr2 film deposited on FTO/TiO2 substrate loaded with PEAI species exhibits larger grain sizes. The statistical results in Fig. S4 indicate its average grain size of ~ 576 nm, which is slightly larger than the one deposited on FTO/TiO2 substrate without loading of PEAI species (~ 475 nm). It should be noted that the formation of coarsened grains is mainly attributed to the decreased nucleation density of CsPbIBr2 grains, as a result of improved non-wetting of CsPbIBr2 precursor to FTO/TiO2 substrate induced by PEAI species (Fig. S5) [36, 43], along with the templating effect of PEAI species during the crystallization of CsPbIBr2 grains.
Figures 2c, d and S6 provide cross-sectional SEM images of the concerned CsPbIBr2 films. They reveal that the films have a similar thickness of ~ 400 nm. And, each of individual grains in the films is able to vertically penetrate entire film thickness. Such feature maybe endow excellent transport behavior of charge carriers along them, since the transport and transfer of charge carriers do so without traversing any grain boundaries in this case [4, 41]. By comparison, interfacial microstructure of these two films differs obviously. For the CsPbIBr2 film deposited on FTO/TiO2 substrate without loading PEAI species, the interface between CsPbIBr2 film and FTO/TiO2 substrate is contacted seamlessly. However, there are many voids that have the typical size of ~ 100 nm in the same interface, in terms of CsPbIBr2 film deposited on FTO/TiO2 substrate loaded with PEAI species. These voids give rise to the rough contact interface between CsPbIBr2 film and FTO/TiO2 substrate. It is also noted that because PEAI species are sublimated during the thermal annealing stage of CsPbIBr2 film, we conclude that the pre-deposited PEAI species likely act as pore-forming agent in the present case. The strategy of using pore-forming agent has been widely used to create pores in traditional ceramic materials [44, 45]. Our viewpoint can be further supported by the cross section SEM image of the same CsPbIBr2 film prepared with large concentration of PEAI solution (10 mg mL−1), as provided in Fig. S7. It is clear that the voids with much larger sizes are produced when the more PEAI species were pre-deposited. Overall, the PEAI species on FTO/TiO2 substrate, serving as pore-forming agent, can promote the formation of large-sized grains in ultimate CsPbIBr2 film as well as some voids at the interface between CsPbIBr2 film and FTO/TiO2 substrate.
3.3 Crystalline and Optical Property Analysis
Next, the crystalline structure and optical properties of as-prepared CsPbIBr2 films are investigated. For clarification, the CsPbIBr2 film as well the cell deposited on FTO/TiO2 substrate without loading of PEAI species are labeled as “without void,” and the ones on FTO/TiO2 substrate with loading of PEAI species are named as “with voids” hereinafter. Figure 3a presents X-ray diffraction (XRD) patterns of CsPbIBr2 films without and with interfacial voids. Both samples exhibit the pure cubic perovskite phase, since the diffraction peaks at 15.1°, 21.4°, 26.6°, 30.4° 33.8°, and 37.8° are well consistent with those of (100), (110), (111), (200), (210), and (211) planes of CsPbIBr2 crystals reported previously [10, 26]. Meanwhile, the XRD peaks of (100) and (200) planes are dominantly intense for both samples, indicating that the grains in them have (100)-preferential orientation. For cubic perovskite polycrystalline films, {100} grains facets are generally along the direction perpendicular to FTO/TiO2 substrate, which well coincides the vertically penetrated grains morphology observed in the cros section SEM images in Figs. 2c, d and S6 for the concerned CsPbIBr2 films. In addition, the dominant diffraction peaks of CsPbIBr2 film with interfacial voids are more intense than the one without voids, and the full-width-at-half-maximum (FWHM) values of its (100) and (200) XRD peaks are smaller (Table S1). These results reveal the better crystallinity with fewer intragranular defects and less scattering of grain boundaries for the CsPbIBr2 film with interfacial voids, in consideration of the similar thickness of the two films [22, 41, 43]. Such features are in accordance with the larger grains of CsPbIBr2 film with interfacial voids.
Figure 3b presents the ultraviolet–visible (UV–vis) spectra of as-prepared CsPbIBr2 films. Both of them exhibit the typical absorption features of CsPbIBr2 materials including the exciton absorption peak at ~ 560 nm and absorption edge of ~ 599 nm that is in accordance with the bandgap of CsPbIBr2 (2.07 eV) [20, 25, 27]. In comparison, the CsPbIBr2 film with interfacial voids shows higher absorption intensities in the whole absorption ranges, revealing its better light absorption capacity. In principle, this desired feature mainly originates from either improved quality of CsPbIBr2 film or scattering effect of interfacial voids in it, in view of the similar thickness of the two films. To exclude the effect of the former, UV–vis characters of the concerned CsPbIBr2 films were collected from the side of FTO glass, wherein the detection beam streams from the top surface of CsPbIBr2 films. The results in Fig. S8 reveal that the differences of their absorption intensities decrease accordingly. That is to say, the better absorption ability of CsPbIBr2 film with interfacial voids observed in Fig. 3b largely comes from the beneficial scattering effect of interfacial voids, which may make great contribution to Jsc of ultimate PSC.
Figure 3c, d gives steady-state photoluminescence (PL) spectra and time-resolved PL (TRPL) curves of CsPbIBr2 films without and with interfacial voids grown on insulating glass substrates instead of FTO/TiO2 substrates, respectively. As shown in Fig. 3c, CsPbIBr2 films exhibit the dominant PL peaks at ~ 600 nm, in well agreement with their bandgaps. The shoulder peaks can be also observed for both samples, as indicated by the logarithmic-scale PL plots in Fig. S9. This phenomenon is mainly caused by light-induced halide phase segregation in CsPbIBr2 films, wherein some I-rich domains with low bandgap and Br-rich domains with wide bandgap are formed under illumination, as demonstrated in previous works [19, 46,47,48]. In addition, the CsPbIBr2 film with interfacial voids yields the stronger PL peak intensity, revealing the weaker non-radiative recombination of charge carriers in it. Figure 3d shows the corresponding TRPL results, which can provide semi-quantitative information about non-radiative recombination of charge carriers [2, 4, 9, 48]. The curves are fitted by a bi-exponential decay function of time (t) [2, 4]: \(F(t) = \sum {A_{i} e^{{\tau /\tau^{i} }} ;\,i = 1,2}\), in which Ai and ti are weight factor and time constant, respectively. In this manner, the average carrier lifetime (tave) can be estimated according to the equation of \(\tau_{\text{ave}} = \sum {A_{i} \tau_{i}^{2} } /\sum {A_{i} \tau_{i} } ;\,i = 1,2\). The CsPbIBr2 film with interfacial voids exhibits the τave of 1.91 ns, which is higher than the one without interfacial voids (1.26 ns). The longer τave of the former indicates the fewer defects in it, which is originally attributed to the larger grains and better crystallinity of CsPbIBr2 film with interfacial voids.
3.4 Photovoltaic Performance
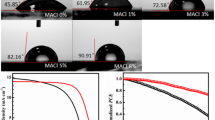
Hitherto, we reveal that the CsPbIBr2 film with interfacial voids has slightly larger grains and higher crystallinity, which result in the weaker non-radiative recombination of charge carriers in it. Such feature is expected to improve the Voc and FF of ultimate PSC [18, 30, 32, 49]. Meanwhile, the interfacial voids trigger intense light scattering effect [50], which gives rise to the much higher absorption of the CsPbIBr2 film and hence maybe boost the Jsc of corresponding PSC. It is generally recognized that interfacial voids are harmful to the performance of planar PSC, since they inevitably reduce the contact area between absorber film and charge transporting layer, thus increasing the transporting resistance of charge carriers. Even so, in the present case, we speculate that such negative effect can be fully counteracted by the multiply beneficial contributions of the interfacial voids described above. To verify our inference, the CsPbIBr2 films without and with interfacial voids are used to construct carbon-based, all-inorganic PSCs with the structure of FTO/TiO2/CsPbIBr2/Carbon as illustrated in Fig. 4a. Firstly, the PEAI solution concentration was optimized according to the performance of resulting PSCs. As shown in Fig. 4b, the PSC yields the optimized PCE when 3 mg mL−1 PEAI solution was used. So, this solution is adopted to fabricate all the PSCs hereinafter. Figure 4c provides the statistical PCEs of 20 independent PSCs based on the CsPbIBr2 films without and with interfacial voids, which were measured under simulated AM 1.5 G illumination (100 mW cm−2) and a scan rate of 10 mV s−1. A black mask containing a circular aperture with specific area of 0.085 cm2 was used to confine the active area of the PSCs. Accordingly, the average PCEs are estimated to be 5.22 ± 0.54% and 9.54 ± 0.59% for the PSCs fabricated with CsPbIBr2 films without and with interfacial voids, respectively. It is extremely surprised that the CsPbIBr2 films with interfacial voids can enable over 85% enhancement in average PCE of carbon-based, all-inorganic PSCs. Figure 4d shows the statistical Voc values of as-fabricated PSCs, from which the average Voc values are estimated to be 1.230 ± 0.023 and 1.297 ± 0.018 V for the PSCs based on CsPbIBr2 films without and with interfacial voids, respectively. Thus, we can conclude that the Voc values of the PSCs based on CsPbIBr2 films with interfacial voids are larger consistently. It is worth noting that the superior Voc is not only vital to overcome the PCE obstacle facing current CsPbIBr2 PSCs, but also can promote their applications in other energy conversion systems, such as water splitting cells [11, 17, 28].
a Schematic layer stacking of carbon-based, all-inorganic CsPbIBr2 PSC, b typical J–V curves of the PSCs employed CsPbIBr2 films prepared with different concentrations of PEAI solutions, c statistic PCEs and d Voc distributions of 20 independent PSCs based on CsPbIBr2 films without and with interfacial voids, e J–V curves, f EQE spectra, and g steady-state photocurrent outputs of the champion PSCs based on CsPbIBr2 films without and with interfacial voids. Time-dependent variations of PCEs of the un-encapsulated PSC based on CsPbIBr2 film with interfacial voids in h glovebox atmosphere and i ambient condition
Figure 4e displays the current density versus voltages (J–V) curves of the best-performing PSCs employed CsPbIBr2 films without and with interfacial voids, respectively. The typical photovoltaic parameters including Voc, FF, Jsc, and PCE are provided in Table 1. The PSC fabricated with CsPbIBr2 film with interfacial voids delivers the Voc of 1.338 V, FF of 0.65, and Jsc of 11.73 mA cm−2, leading to a much higher PCE of 10.20%. Such PCE is beyond nearly all the CsPbIBr2 PSCs reported previously. More importantly, as shown in Table 2, the Voc achieved herein stands the highest value among them [8, 17,18,19,20, 25,26,27, 30, 31, 35, 47, 48, 51,52,53,54,55,56].
External quantum efficiency (EQE) spectra of the champion PSCs are given in Fig. 4f. Photocurrent response of each PSC is consistent with the absorption feature of CsPbIBr2 film. By contrast, the PSC based on CsPbIBr2 film with interfacial voids exhibits much higher EQEs in the wavelength regions from 400 to 580 nm, disclosing its better photoelectric conversion capability as a result of higher light absorption and more energetic extraction of charge carriers. Moreover, a remarkable EQE peak of 86% can be realized in the PSC. In addition, the integrated current densities from the EQE spectra (Fig. S10) are basically consistent with the Jsc provided in Table 1, wherein the discrepancies may be resulted from the absence of light soaking during EQE tests and the spectral mismatch [30, 31, 52].
We also tested the steady-state photocurrent outputs of the champion PSCs under maximum power-point conditions (Fig. 4g). They exhibit fast light-response, indicating smooth charge extraction. This desired character is mainly relied to large energy difference of conduction band minimums (CBMs) for CsPbIBr2 absorber and TiO2 ETL [18, 51]. One can see that the PSC based on CsPbIBr2 film without interfacial voids yields the stabilized current density of ~ 6.18 mA cm−2 under a constant bias voltage of 0.880 V, which corresponds to a stabilized PCE of 5.44%. And, the one without interfacial voids exhibits the stabilized PCE of 8.99% under 1.040 V, which supports the better performance of the PSC fabricated with CsPbIBr2 film with interfacial voids. However, both steady-state PCEs are smaller than those of extracted from J–V curves, indicative of inevitable anomalous J–V hysteresis. The J–V hysteresis is frequently observed in previous CsPbIBr2 PSCs and primarily related to light-induced phase segregation in CsPbIBr2 films [8, 46,47,48, 51, 52, 54, 55]. In addition, one can notice that during the test period the photocurrent outputs of both PSCs are stable in ambient atmosphere, revealing their excellent ability against humidity and light attacking.
Finally, thermal stability and long-term stability of the champion PSC prepared with CsPbIBr2 film with interfacial voids were studied. For thermal stability test, the un-encapsulated PSC was continuously heated at 85 °C on a hotplate in N2-filled glovebox. And, for long-term stability test the same PSC was stored at room temperature in ambient atmosphere with relative humidity of 30–40%. As shown in Fig. 4h, the PSC can remain ~ 97% of its initial PCE after being tested for 12 h, indicative of its superior thermal stability. We attribute this favored aspect to the excellent resistance of CsPbIBr2 film to high temperature, as it is prepared at a higher annealing temperature of 280 °C. In general, the annealing temperatures for its analogs including CsPbI3, CsPbI2Br, and CsPbBr3 are 200, 160, and 250 °C, respectively. Figure 4i provides the result of long-term stability test. The same PSC can sustain ~ 94% of its initial PCE after storing for 60 days, making clear its superior long-term stability against humidity and interface degradation. Overall, one can conclude that the CsPbIBr2 film with interfacial voids can enable the carbon-based, all-inorganic PSC with outstanding PCE and record-high Voc, along with excellent operation stability under light, thermal, or humidity stress.
3.5 Carrier Dynamics
To gain insight into the relationship between the carrier dynamics and performance of PSCs prepared with CsPbIBr2 films without and with interfacial voids, we explored the transport mechanisms in the fabricated PSCs by means of various characterization techniques, including dark J–V, light-intensity-dependent Voc, transient photocurrent (TPC), transient photovoltage (TPV), electrochemical impedance spectroscopy (EIS), and Mott–Schottky (M–S) measurements. Figure 5a gives the dark J–V curves of investigated PSCs. In general, the dark J–V curve of a solar cell is dominated by the diode law. Based on it, the J0 can be estimated from the intercept at zero voltage of the linear regression from semi-logarithmic J–V plot in the region close to the diode turn-on voltage [19, 23, 57]. In general, J0 is governed by recombination of charge carriers in the cell, wherein a low J0 is always companied by the suppressed recombination. Herein, we can see that the PSC based on CsPbIBr2 film with interfacial voids exhibits much smaller J0 (1.14 × 10−7 mA cm−2) than the one without interfacial voids (1.37 × 10−5 mA cm−2), indicating the weaker recombination of charge carriers in it. It should be noted that although the larger grains and higher crystallinity can partly decrease J0 of the PSC based on CsPbIBr2 film with interfacial voids, we believe that the interfacial voids may dominantly contribute to its much smaller J0. This is because the PSC exhibits the similarly lower dark current under high bias voltages such as 1.5 V.
Figure 5b presents the plots of Voc as a function of incident light intensity for the concerned PSCs. One can see that the Voc improves with the logarithmic light intensity monotonically, revealing the dominant trap-assisted Shockley–Read–Hall recombination in both PSCs [9, 23, 30, 31]. In addition, the PSC based on CsPbIBr2 film without interfacial void shows a slope of 2.08 kBT/q, while the one with voids yields a smaller slope of 1.70 kBT/q that is more close to 1 kBT/q, where q is the electric charge, T is temperature, and kB is the Boltzmann constant. These results further confirm that the recombination of charge carries has been greatly suppressed in the PSC based on CsPbIBr2 film with interfacial voids.
Figure 5c gives the TPC curves of the corresponding PSCs, which can reflect the extraction and transport properties of charge carriers. The photocurrent decay constants are estimated to be 2.64 and 1.83 μs for the PSCs based on CsPbIBr2 film without and with interfacial voids, respectively. These results mean that the interfacial voids do not damage the extraction and transport of charge carriers [18, 19, 51], even though they induce significantly decreased dark current density. Instead, the extraction and transport of charge carriers are improved slightly, which probably benefit from the larger grains and higher crystallinity of CsPbIBr2 film with interfacial voids that endow its better transport ability for photon-generated carriers. At the same time, the recombination of charge carriers in the PSCs is monitored by TPV measurements [18, 19, 51]. The results in Fig. 5d indicate that the PSC based on CsPbIBr2 film with interfacial voids has the slower photovoltage decay (1.06 ms) than the one without interfacial void (0.79 ms), which supports the suppressed recombination of charge carriers in the former again.
Furthermore, EIS tests were performed on the fabricated PSCs to estimate recombination resistance (Rrec) of charge carriers in them [14, 17, 25]. The Nyquist plots that were recorded at a forward bias voltage of 1.0 V under dark condition are given in Fig. 5e. The clear arc is involved to carrier recombination, reflecting the recombination resistance Rrec. The larger Rrec of the PSC based on CsPbIBr2 film with interfacial voids is well accordance with the suppressed recombination of charge carriers in it. On the other hand, the M–S results in Fig. 5f indicate that the built-in potential (Vbi) of the PSC based on CsPbIBr2 film with interfacial voids is 1. 56 V, which is much higher than that of the one without interfacial void (Vbi = 1.30 V). A larger Vbi not only provide higher driving force to dissociate photo-generated carriers, but also contribute to form a wider depletion region for suppressing recombination of charge carriers in the PSC, as verified by the dark J–V, TPV, and EIS results above [15, 18, 30, 31]. As a whole, the higher Vbi and weaker recombination of charge carriers directly contributes to the record-high Voc as well as superior FF of the PSC based on CsPbIBr2 film with interfacial voids. And, the higher absorption of CsPbIBr2 film as a result of intense light scattering effect of interfacial voids gives rise to its higher Jsc. Therefore, the PCE of the PSC based on CsPbIBr2 film with interfacial voids is improved significantly.
4 Conclusions
We demonstrate a new interfacial engineering for carbon-based, inorganic CsPbIBr2 PSC by manufacturing interfacial voids between TiO2 ETL and CsPbIBr2 film. The interfacial voids contribute to better absorption, higher Vbi, and smaller J0 of the PSC, as results of enhanced light scattering in CsPbIBr2 film, boosted crystal quality of CsPbIBr2 grains, and significant suppressed recombination of charge carriers. Consequently, the optimized PSC achieves the PCE of as high as 10.20% coupled with the Voc of 1.338 V. Such Voc represents the best value among CsPbIBr2 PSCs reported earlier. More promisingly, the non-encapsulated PSC can remain ~ 97% and ~ 94% of its initial PCE after being heated at 85 °C for 12 h or storing in ambient atmosphere with relative humidity of 30–40% for 60 days, respectively, suggesting its excellent stability against light, thermal, and humidity stresses.
References
A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). https://doi.org/10.1021/ja809598r
H.S. Jung, N.G. Park, Perovskite solar cells: from materials to devices. Small 11, 10–25 (2015). https://doi.org/10.1002/smll.201402767
Y. Rong, Y. Hu, A. Mei, H. Tan, M.I. Saidaminov et al., Challenges for commercializing perovskite solar cells. Science 361, eaat8235 (2018). https://doi.org/10.1126/science.aat8235
M. Saliba, J.P. Correa-Baena, M. Grätzel, A. Hagfeldt, A. Abate, Perovskite solar cells: from the atomic level to film quality and device performance. Angew. Chem. Int. Ed. 57, 2554–2569 (2018). https://doi.org/10.1002/anie.201703226
Cell efficiency chart. https://www.nrel.gov/pv/cell-efficiency.html. Accessed: Aug 2019
C.C. Boyd, R. Cheacharoen, T. Leijtens, M.D. McGehee, Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem. Rev. 119, 3418–3451 (2019). https://doi.org/10.1021/acs.chemrev.8b00336
J. Liang, C. Wang, Y. Wang, Z. Xu, Z. Lu et al., All-inorganic perovskite solar cells. J. Am. Chem. Soc. 138, 15829–15832 (2016). https://doi.org/10.1021/jacs.6b10227
J. Liang, P. Zhao, C. Wang, Y. Wang, Y. Hu, G. Zhu, L. Ma, J. Liu, Z. Jin, CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 139, 14009–14012 (2017). https://doi.org/10.1021/jacs.7b07949
J. Duan, Y. Zhao, B. He, Q. Tang, High-purity inorganic perovskite films for solar cells with 9.72% efficiency. Angew. Chem. Int. Ed. 57, 3787–3791 (2018). https://doi.org/10.1002/anie.201800019
J. Zhang, G. Hodes, Z. Jin, S. Liu, All-inorganic CsPbX3 perovksite solar cells: progress and prospects. Angew. Chem. Int. Ed. 58, 15596–15618 (2019). https://doi.org/10.1002/anie.201901081
A. Ho-Baillie, M. Zhang, C.F.J. Lau, F.-J. Ma, S. Huang, Untapped potentials of inorganic metal halide perovskite solar cells. Joule 3, 938–955 (2019). https://doi.org/10.1016/j.joule.2019.02.002
S. Xiang, Z. Fu, W. Li, Y. Wei, J. Liu et al., Highly air-stable carbon-based α-CsPbI3 perovskite solar cells with a broadened optical spectrum. ACS Energy Lett. 3, 1824–1831 (2018). https://doi.org/10.1021/acsenergylett.8b00820
S. Xiang, W. Li, Y. Wei, J. Liu, H. Liu, L. Zhu, S. Yang, H. Chen, Natrium doping pushes the efficiency of carbon-based CsPbI3 perovskite solar cells to 10.7%. iScience 15, 156 (2019). https://doi.org/10.1016/j.isci.2019.04.025
X. Zhang, Y. Zhou, Y. Li, J. Sun, X. Lu et al., Efficient and carbon-based hole transport layer-free CsPbI2Br planar perovskite solar cells using PMMA modification. J. Mater. Chem. C 7, 3852–3861 (2019). https://doi.org/10.1039/C9TC00374F
Y. Guo, F. Zhao, J. Tao, J. Jiang, J. Zhang, J. Yang, Z. Hu, J. Chu, Efficient and hole-transporting-layer-free CsPbI2Br planar heterojunction perovskite solar cells through rubidium passivation. ChemSusChem 12, 983–989 (2019). https://doi.org/10.1002/cssc.201802690
X. Meng, Z. Wang, W. Qian, Z. Zhu, T. Zhang et al., Excess cesium iodide induces spinodal decomposition of CsPbI2Br perovskite films. J. Phys. Chem. Lett. 10, 194–199 (2019). https://doi.org/10.1021/acs.jpclett.8b03742
Z. Guo, S. Teo, Z. Xu, C. Zhang, Y. Kamata, S. Hayase, T. Ma, Achievable high Voc of carbon based all-inorganic CsPbIBr2 perovskite solar cells through interface engineering. J. Mater. Chem. A 7, 1227–1232 (2019). https://doi.org/10.1039/C8TA09838G
W. Zhu, Z. Zhang, W. Chai, Q. Zhang, D. Chen et al., Band alignment engineering towards 10.71%-efficiency carbon-based, all-inorganic planar CsPbIBr2 perovskite solar cells. ChemSusChem 12, 2318 (2019). https://doi.org/10.1002/cssc.201900611
W. Zhu, Q. Zhang, C. Zhang, Z. Zhang, D. Chen, Z. Lin, J. Chang, J. Zhang, Y. Hao, Aged precursor solution toward low-temperature fabrication of efficient carbon-based all-inorganic planar CsPbIBr2 perovskite solar cells. ACS Appl. Energy Mater. 1, 4991–4997 (2018). https://doi.org/10.1021/acsaem.8b00972
J. Liang, Z. Liu, L. Qiu, Z. Hawash, L. Meng et al., Enhancing optical, electronic, crystalline, and morphological properties of cesium lead halide by Mn substitution for high-stability all-inorganic perovskite solar cells with carbon electrodes. Adv. Energy Mater. 8, 1800504 (2018). https://doi.org/10.1002/aenm.201800504
X. Liu, X. Tan, Z. Liu, H. Ye, B. Sun, T. Shi, Z. Tang, G. Liao, Boosting the efficiency of carbon-based planar CsPbBr3 perovskite solar cells by a modified multistep spin-coating technique and interface engineering. Nano Energy 56, 184–195 (2019). https://doi.org/10.1016/j.nanoen.2018.11.053
J. Liu, L. Zhu, S. Xiang, Y. Wei, M. Xie, H. Liu, W. Li, H. Chen, Growing high-quality CsPbBr3 by using porous CsPb2Br5 as an intermediate: a promising light absorber in carbon-based perovskite solar cells. Sustain. Energy Fuels 3, 184–194 (2019). https://doi.org/10.1039/C8SE00442K
J. Duan, Y. Zhao, X. Yang, Y. Wang, B. He, Q. Tang, Lanthanide ions doped CsPbBr3 halides for HTM-free 10.14%-efficiency inorganic perovskite solar cell with an ultrahigh open-circuit voltage of 1.594 V. Adv. Energy Mater. 8, 1802346 (2018). https://doi.org/10.1002/aenm.201802346
Y. Zhou, Y. Zhao, Chemical stability and instability of inorganic halide perovskites. Energy Environ. Sci. 12, 1495 (2019). https://doi.org/10.1039/C8EE03559H
Z. Wang, A.K. Baranwal, M.A. Kamarudin, C.H. Ng, M. Pandey, T. Ma, S. Hayase, Xanthate-induced sulfur doped all-inorganic perovskite with superior phase stability and enhanced performance. Nano Energy 59, 258–267 (2019). https://doi.org/10.1016/j.nanoen.2019.02.049
J. Lin, M. Lai, L. Dou, C.S. Kley, H. Chen et al., Thermochromic halide perovskite solar cells. Nat. Mater. 17, 261 (2018). https://doi.org/10.1038/s41563-017-0006-0
B. Yang, M. Wang, X. Hu, T. Zhou, Z. Zang, Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low-temperature processed In2S3 as electron-transport-layer. Nano Energy 57, 718–727 (2019). https://doi.org/10.1016/j.nanoen.2018.12.097
M. Moniruddin, B. Ilyassov, X. Zhao, E. Smith, T. Serikov, N. Ibrayev, R. Asmatulu, N. Nuraje, Recent progress on perovskite materials in photovoltaic and water splitting applications. Mater. Today Energy 7, 246–259 (2018). https://doi.org/10.1016/j.mtener.2017.10.005
F. Meillaud, A. Shah, C. Droz, E. Vallat-Sauvain, C. Miazza, Efficiency limits for single-junction and tandem solar cells. Sol. Energy Mater. Sol. Cells 90, 2952–2959 (2006). https://doi.org/10.1016/j.solmat.2006.06.002
W.S. Subhani, K. Wang, M. Du, X. Wang, S. Liu, Interface-modification-induced gradient energy band for highly efficient CsPbIBr2 perovskite solar cells. Adv. Energy Mater. (2019). https://doi.org/10.1002/aenm.201803785
W.S. Subhani, K. Wang, M. Du, S.F. Liu, Gold Schmidt-rule-deviated perovskite CsPbIBr2 by barium substitution for efficient solar cells. Nano Energy 61, 165–172 (2019). https://doi.org/10.1016/j.nanoen.2019.04.066
F. Meng, A. Liu, L. Gao, J. Cao, Y. Yan et al., Current progress in interfacial engineering of carbon-based perovskite solar cells. J. Mater. Chem. A 7, 8690–8699 (2019). https://doi.org/10.1039/C9TA01364D
D.B. Khadka, Y. Shirai, M. Yanagida, K. Miyano, Degradation of encapsulated perovskite solar cells driven by deep trap states and interfacial deterioration. J. Mater. Chem. C 6, 162–170 (2018). https://doi.org/10.1039/C7TC03733C
B. Roose, Q. Wang, A. Abate, The role of charge selective contacts in perovskite solar cell stability. Adv. Energy Mater. 9, 1803140 (2019). https://doi.org/10.1002/aenm.201803140
C. Liu, W. Li, J. Chen, J. Fan, Y. Mai, R.E. Schropp, Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr2 perovskite solar cells. Nano Energy 41, 75–83 (2017). https://doi.org/10.1016/j.nanoen.2017.08.048
C. Bao, J. Yang, S. Bai, W. Xu, Z. Yan et al., High performance and stable all-inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 30, 1803422 (2018). https://doi.org/10.1002/adma.201803422
Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen et al., Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 13, 460–466 (2019). https://doi.org/10.1038/s41566-019-0398-2
Y. Wang, T. Zhang, M. Kan, Y. Zhao, Bifunctional stabilization of all-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 140, 12345–12348 (2018). https://doi.org/10.1021/jacs.8b07927
A. Dualeh, P. Gao, S.I. Seok, M.K. Nazeeruddin, M. Grätzel, Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem. Mater. 26, 6160–6164 (2014). https://doi.org/10.1021/cm502468k
N.N. Shlenskaya, N.A. Belich, M. Grätzel, E.A. Goodilin, A.B. Tarasov, Light-induced reactivity of gold and hybrid perovskite as a new possible degradation mechanism in perovskite solar cells. J. Mater. Chem. A 6, 1780–1786 (2018). https://doi.org/10.1039/C7TA10217H
W. Zhu, C. Bao, B. Lv, F. Li, Y. Yi et al., Dramatically promoted crystallization control of organolead triiodide perovskite film by a homogeneous cap for high efficiency planar-heterojunction solar cells. J. Mater. Chem. A 4, 12535–12542 (2016). https://doi.org/10.1039/C6TA04332A
G. Gottstein, A. King, L. Shvindlerman, The effect of triple-junction drag on grain growth. Acta Mater. 48, 397–403 (2000). https://doi.org/10.1016/S1359-6454(99)00373-0
C. Bi, Q. Wang, Y. Shao, Y. Yuan, Z. Xiao, J. Huang, Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 6, 7747 (2015). https://doi.org/10.1038/ncomms8747
F. Chen, Q. Shen, F. Yan, L. Zhang, Pressureless sintering of α-Si3N4 porous ceramics using a H3PO4 pore-forming agent. J. Am. Ceram. Soc. 90, 2379–2383 (2007). https://doi.org/10.1111/j.1551-2916.2007.01800.x
C. Galassi, Processing of porous ceramics: piezoelectric materials. J. Eur. Ceram. Soc. 26, 2951–2958 (2006). https://doi.org/10.1016/j.jeurceramsoc.2006.02.011
D.J. Slotcavage, H.I. Karunadasa, M.D. McGehee, Light-induced phase segregation in halide-perovskite absorbers. ACS Energy Lett. 1, 1199–1205 (2016). https://doi.org/10.1021/acsenergylett.6b00495
W. Li, M.U. Rothmann, A. Liu, Z. Wang, Y. Zhang et al., Phase segregation enhanced ion movement in efficient inorganic CsPbIBr2 solar cells. Adv. Energy Mater. 7, 1700946 (2017). https://doi.org/10.1002/aenm.201700946
N. Li, Z. Zhu, J. Li, A.K.Y. Jen, L. Wang, Inorganic CsPb1−xSnxIBr2 for efficient wide-bandgap perovskite solar cells. Adv. Energy Mater. 8, 1800525 (2018). https://doi.org/10.1002/aenm.201800525
I. Gelmetti, N. Montcada, A. Perez-Rodriguez, E. Barrena, C. Ocal et al., Energy alignment and recombination in perovskite solar cells: weighted influence on the open circuit voltage. Energy Environ. Sci. 12, 1309–1316 (2019). https://doi.org/10.1039/C9EE00528E
S.M. Kang, S. Jang, J.K. Lee, J. Yoon, D.E. Yoo, J.W. Lee, M. Choi, N.G. Park, Moth-Eye TiO2 layer for improving light harvesting efficiency in perovskite solar cells. Small 12, 2443–2449 (2016). https://doi.org/10.1002/smll.201600428
Q. Zhang, W. Zhu, D. Chen, Z. Zhang, Z. Lin et al., Light processing enables efficient carbon-based, all-inorganic planar CsPbIBr2 solar cells with high photovoltages. ACS Appl. Mater. Interfaces 11, 2997–3005 (2018). https://doi.org/10.1021/acsami.8b17839
Y. Jiang, J. Yuan, Y. Ni, J. Yang, Y. Wang, T. Jiu, M. Yuan, J. Chen, Reduced-dimensional α-CsPbX3 perovskites for efficient and stable photovoltaics. Joule 2, 1356–1368 (2018). https://doi.org/10.1016/j.joule.2018.05.004
J. Lu, S.-C. Chen, Q. Zheng, Defect passivation of CsPbIBr2 perovskites for high-performance solar cells with large open-circuit voltage of 1.28 V. ACS Appl. Energy Mater. 1, 5872–5878 (2018). https://doi.org/10.1021/acsaem.8b01430
C.F.J. Lau, X. Deng, Q. Ma, J. Zheng, J.S. Yun, M.A. Green, S. Huang, A.W. Ho-Baillie, CsPbIBr2 perovskite solar cell by spray-assisted deposition. ACS Energy Lett. 1, 573–577 (2016). https://doi.org/10.1021/acsenergylett.6b00341
M. Aamir, T. Adhikari, M. Sher, N. Revaprasadu, W. Khalid, J. Akhtar, J.-M. Nunzi, Fabrication of planar heterojunction CsPbBr2I perovskite solar cells using ZnO as an electron transport layer and improved solar energy conversion efficiency. New J. Chem. 42, 14104–14110 (2018). https://doi.org/10.1039/C8NJ02238K
Q. Ma, S. Huang, X. Wen, M.A. Green, A.W. Ho-Baillie, Hole transport layer free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation. Adv. Energy Mater. 6, 1502202 (2016). https://doi.org/10.1002/aenm.201502202
C. Li, Z. Song, D. Zhao, C. Xiao, B. Subedi et al., Reducing saturation-current density to realize high-efficiency low-bandgap mixed tin-lead halide perovskite solar cells. Adv. Energy Mater. 9(3), 1803135 (2019). https://doi.org/10.1002/aenm.201803135
Acknowledgements
All the authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 61804113, 61874083), Initiative Postdocs Supporting Program (BX20190261), the National Natural Science Foundation of Shaanxi Province (2018ZDCXL-GY-08-02-02 and 2017JM6049), and the Fundamental Research Funds for the Central Universities (JB181107 and JBX171103).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, W., Zhang, Z., Chen, D. et al. Interfacial Voids Trigger Carbon-Based, All-Inorganic CsPbIBr2 Perovskite Solar Cells with Photovoltage Exceeding 1.33 V. Nano-Micro Lett. 12, 87 (2020). https://doi.org/10.1007/s40820-020-00425-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-020-00425-1