Abstract
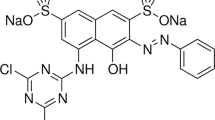
The use of biomass residues from agricultural processes for the production of cheap and competitive activated carbon (AC) is an excellent option to minimize the costs of existing procedures to remove dyes from wastewater. In this study, the potential use of AC obtained from peanut hulls and rice husks for adsorption of Cibacron Yellow F-4G (CYF-4G) is examined. The activated peanut hull (PHAC) and rice husk (RHAC) were characterized by TGA, FTIR, BET and elemental analysis. The effects of different process variables as well as the dose of adsorbent, dye concentration and pH were evaluated. A decrease in amount of dye adsorbed per unit adsorbent mass was observed when increasing CYF-4G concentration. The results showed an optimal dye adsorption value at a pH of 2.0. The adsorption kinetics of CYF-4G are governed by the pseudo-second-order model. In addition, adsorption fits the Langmuir isotherm better than Freundlich’s. Adsorption capacities of AC prepared from agricultural waste show that PHAC performs better than RHAC to remove CYF-4G.














Similar content being viewed by others
References
Saraswathi K, Balakumar S (2009) Biodecolourization of azodye (pigmented red 208) using Bacillus firmus and Bacillus laterosporus. J Biosci Tech 1:1–7
Sen TK, Afroze S, Ang HM (2011) Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiate. Water Air Soil Pollut 218:499–515
Yao Z, Wang L, Qi J (2009) Biosorption of methylene blue from aqueous solution using a bioenergy forest waste: Xanthoceras sorbifolia seed coat. Clean Soil Air Water 37(8):642–648
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119
Cheunbarn T, Cheunbarn S, Khumjai T (2008) Prospects of bacterial granule for treatment of real textile industrial wastewater. Int J Agric Biol 10(6):89–92
Dubey SK, Pandey A, Bajaj AK, Misra K (2007) Some commercial azo dyes as inhibitors of mushroom tyrosinase DOPA oxidase activity. J Pharmacol Toxicol 2:718–724
Pinheiro HM, Touraud E, Thomas O (2004) Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigments 61:121–139
Sohrabi MR, Ghavami M (2008) Photocatalytic degradation of direct red 23 dye using UV/TiO2: effect of operational parameters. J Hazard Mater 153:1235–1239
Abbasi M, Asl NR (2008) Sonochemical degradation of basic blue 41 dye assisted by nanoTiO2 and H2O2. J Hazard Mater 153:942–947
Zaghbani N, Hafiane A, Dhahbi M (2008) Removal of Safranin T from wastewater using micellar enhanced ultrafiltration. Desalination. 222:348–356
Wu JS, Liu CH, Chu KH, Suen SY (2008) Removal of cationic dye methyl violet 2B from water by cation exchange membranes. J Membr Sci 309:239–245
Fan L, Zhou Y, Yang W, Chen G, Yang F (2008) Electrochemical degradation of aqueous solution of Amaranth azo dye on ACF under potentiostatic model. Dyes Pigments 76:440–446
Zhu MX, Lee L, Wang HH, Wang Z (2007) Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J Hazard Mater 149:735–741
Sudarjanto G, Keller-Lehmann B, Keller J (2006) Optimization of integrated chemical-biological degradation of a reactive azo dye using response surface methodology. J Hazard Mater 138:160–168
Garcia-Montano J, Pérez-Estrada L, Oller I, Maldonado MI, Torrades F, Peral J (2008) Pilot plant scale reactive dyes degradation by solar photo-Fenton and biological processes. J Photochem Photobiol A 195:205–214
Lodha B, Chaudhari S (2007) Optimization of Fenton-biological treatment scheme for the treatment of aqueous dye solutions. J Hazard Mater 148:459–466
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sust Energ Rev 46:218–235
Tsai WT, Chang CY, Lin MC, Chien SF, Sun HF, Hsieh MF (2001) Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere. 45:51–58
Senthilkumar S, Varadarajan PR, Porkodi K, Subbhuraam CV (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284:78–82
Tan IA, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–347
Hameed BH, Ahmad AL, Latiff KNA (2007) Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes Pigments 75:143–149
Thinakaran N, Baskaralingam P, Pulikesi M, Panneerselvam P, Sivanesan S (2008) Removal of acid violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J Hazard Mater 151:316–322
Theivarasu C, Mylsamy S, Sivakumar N (2011) Studies on the removal of reactive orange 16 from aqueous solution using cocoa shell carbon as an adsorbent. Indian J Environ Prot 31(7):588–594
Lupul I, Yperman J, Carleer R, Gryglewicz G (2015) Tailoring of porous texture of hemp stem-based activated carbon produced by phosphoric acid activation in steam atmosphere. J Porous Mater 22:283–289
Noorimotlagh Z, Soltani RDC, Khataee AR, Shahriyar S, Nourmoradi H (2014) Adsorption of a textile dye in aqueous phase using mesoporous activated carbon prepared from Iranian milk vetch. J Taiwan Inst Chem Eng 45(4):1783–1791
Noorimotlagh Z, Shahriyar S, Darvishi Cheshmeh Soltani R, Tajik R (2016) Optimized adsorption of 4-chlorophenol onto activated carbon derived from milk vetch utilizing response surface methodology. Desalin Water Treat 57(30):14213–14226
Curbelo A, Garcia B (2017) Contribución de la biomasa no cañera a la generación de electricidad en Cuba. División de Industria y Energía, Agencia de Ciencia y Tecnología, Cuba. From (November 1, 2019): http://www.fao.org/3/t2363s/t2363s0i.htm
Fundora MZ, Hernandez M, Lopez R, Fernandez L, Sanchez A, Lopez J, Ravelo I (2004) Analysis of the variability in collected peanut (Arachis hypogaea L.) cultivars for the establishment of core collections. Plant Genet Res Newsl 137:9–13
Hamm W, Hamilton RJ (2000) Edible oil processing. CRC Press, New York, NY, pp 85–86
Martínez M (2017) Paralizan molino arrocero por contaminación ambiental. Manzanillo para el Mundo. From (November 1, 2019): http://mzllompm.blogspot.com/2017/03/accionan-por-contaminacion-ambiental-en.html
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519
Cornelissen T, Yperman J, Reggers G, Schreurs S, Carleer R (2008) Flash co-pyrolysis of biomass with polylactic acid. Part 1: Influence on bio-oil yield and heating value. Fuel. 87(7):1031–1041
Vanreppelen K, Vanderheyden S, Kuppens T, Schreurs S, Yperman J, Carleer R (2014) Activated carbon from pyrolysis of brewer’s spent grain: production and adsorption properties. Waste Manag Res 32(7):634–645
Faria PCC, Orfao JJM, Pereira MFR (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res 38:2043–2052
Boehm HP (1966) Chemical identification of surface groups. In: DD Eley, H Pines, PBWeisz (eds) Adv Catal. Academic Press, pp 179–274
Boehm HP (1994) Some aspects of the surface-chemistry of carbon-blacks and other carbons. Carbon. 32:759–769
Stoeckli F, Daguerre E, Guillot A (1999) The development of micropore volumes and widths during physical activation of various precursors. Carbon. 37:2075–2077
Neimark AV, Lin Y, Ravikovitch PI, Thommes M (2009) Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon. 47:1617–1628
Stoeckli F, Lopez-Ramon MV, Moreno-Castilla C (2001) Adsorption of phenolic compounds from aqueous solutions, by activated carbons, described by the Dubinin−Astakhov equation. Langmuir. 17:3301–3306
Tarazona P, Marini Bettolo Marconi U, Evans R (1987) Phase equilibria of fluid interfaces and confined fluids: non-local versus local density functionals. Mol Phys 60:573
Tran HN, You SJ, Bandegharaei AH, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Davoudinejad M, Ghorbanian SA (2013) Modelling of adsorption isotherm of benzoic compounds onto GAC and introducing three new isotherm models using new concept of adsorption effective surface (AES). Sci Res Essays 8(46):2263–2275
Brouers F, Al-Musawi TJ (2015) On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq 212:46–51
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar. 24:1–39
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv Colloid Interf Sci 152:2–13
Cortés-Martínez R, Martínez-Miranda V, Solache-Ríos M, García-Sosa I (2004) Evaluation of natural and surfactant-modified zeolite in the removal of cadmium from aqueous solutions. Sep Sci Technol 39(11):2711–2730
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135:264–273
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–59
Dogan M, Alkan M (2003) Adsorption kinetics of methyl violet onto perlite. Chemosphere. 50(4):517–528
Lakshmi UR, Srivastava VC, Mall ID, Lataye D (2008) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics of indigo Carline dye. J Environ Manag 90(2):710–720
Kumar K, Porkodi K (2007) Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J Hazard Mater 146(1–2):214–226
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater 134(1–3):257–267
Cheung W, Szeto Y, McKay Y (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98(15):2897–2904
Salman JM, Njoku VO, Hameed BH (2011) Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium. Chem Eng J 173:361–368
Pereira MFR, Soares SF, Órfão JJM, Figueiredo JL (2003) Adsorption of dyes on activated carbons: influence of surface chemical groups. Carbon. 41:811–821
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl Chem 57(4):603–619
Choma J, Jaroniec M (2006) Characterization of nanoporous carbons by using gas adsorption isotherms. In: Bandosz TJ (ed) Activated carbon surfaces in environmental remediation. Elsevier Ltd., NewYork, pp 107–158
Gündüz F, Bayrak B (2017) Biosorption of malachite green from an aqueous solution using pomegranate peel: equilibrium modelling, kinetic and thermodynamic studies. J Mol Liq 243:790–798
Ozacar M, Ayhan Sengil I (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96:791–795
Paul J, Naik DB, Sabharwal S (2010) High energy induced decoloration and mineralization of reactive red 120 dye in aqueous solution: a steady state and pulse radiolysis study. Radiat Phys Chem 79:770–776
Errais E, Duplay J, Elhabiri M, Khodja M, Ocampo R, Baltenweck-Guyot R, Darragi F (2012) Anionic rr120 dye adsorption onto raw clay: surface properties and adsorption mechanism. Colloids Surf A Physicochem Eng Asp 403:69–78
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77:16–23
Acevedo B, Barriocanal C (2015) Simultaneous adsorption of Cd2+ and reactive dye on mesoporous nanocarbons. RSC Adv 5(115):95247–95255
Newcombe G, Drikas M, Hayes R (1997) Influence of characterised natural organic material on activated carbon adsorption: II. Effect on pore volume distribution and adsorption of 2-methylisoborneol. Water Res 31(5):1065–1073
Kyzas GZ, Deliyanni EA, Lazaridis NK (2014) Magnetic modification of microporous carbon for dye adsorption. J Colloid Interface Sci 430:166–173
Namasivayam C, Yamuna RT (1992) Removal of Congo red from aqueous solutions by biogas waste slurry. J Chem Technol Biotechnol 53:153–157
Namasivayam C, Yamuna RT (1993) Colour removal from aqueous solutions by biogas residual slurry. Toxicol Environ Chem 38:131–143
Namasivayam C, Yamuna RT (1994) Utilizing biogas residual slurry for dye adsorption. Am Dyestuff Rep 83(8):22–28
Ossman ME, Abdel Fatah M, Taha NA (2014) Fe(III) removal by activated carbon produced from Egyptian rice straw by chemical activation. Desalin Water Treat 52(16–18):3159–3168
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223
Hadi P, Ying Yeung K, Barford J, Jin An K, McKay G (2015) Significance of “effective” surface area of activated carbons on elucidating the adsorption mechanism of large dye molecules. J Environ Chem Eng 3(2):1029–1037
Zhu HY, Fu YQ, Jiang R, Jiang JH, Xiao L, Zeng GM, Zhao SL, Wang Y (2011) Adsorption removal of Congo red onto magnetic cellulose / Fe3O4 / activated carbon composite: equilibrium, kinetic and thermodynamic studies. Chem Eng 173:494–502
Tamai H, Yoshida T, Sasaki M, Yasuda H (1999) Dye adsorption on mesoporous activated carbon fibre obtained from pitch containing yttrium complex. Carbon 37(6):983–989
Acknowledgements
Authors want to thank the VLIR-UOS project (Flemish Inter University Council Cooperation for Development) in the context of the Institutional University Cooperation Program with Universidad de Oriente, especially by means of the P-5 project “Energy, Biofuel and Clean Technologies for sustainable development in the eastern of Cuba”.
Elsy Thijssen and Martine Vanhamel are acknowledged for FTIR measurements and Guy Reggers for TGA measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Machado Garcia, R., Carleer, R., Arada Pérez, M. et al. Adsorption of Cibacron Yellow F-4G dye onto activated carbons obtained from peanut hull and rice husk: kinetics and equilibrium studies. Biomass Conv. Bioref. 12, 323–339 (2022). https://doi.org/10.1007/s13399-020-00699-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00699-w