Abstract
Performance characteristics of peel of Artocarpus nobilis fruit can be enhanced by the treatment of the biosorbent with aqueous NaOH solutions under stirred conditions, followed by thorough washing of the treated biosorbent with water. A series of experiments performed within a wide range of solution pH demonstrates that the optimum pH for Cr(III) removal is pH 5.0, while it is 2.0 for Cr(VI) by peel of Artocarpus nobilis fruit after treatment with aqueous NaOH solutions of concentrations varied from 0.0050 to 0.15 M. Adsorption of Cr(III) and Cr(VI) from individual aqueous solutions on the above biosorbent treated with 0.010 M NaOH solution, which provides the highest extent of removal of 4.89 × 103 mg kg−1 (pH = 5) and 4.94 × 103 mg kg−1 (pH = 2), respectively, follows pseudo-first-order kinetics at the ambient temperature of 27.5 °C when the concentration of adsorbate concentration is kept relatively constant. The order of the reaction is unchanged when the solution temperature is increased up to 40.0 °C for Cr(III) although the reaction becomes pseudo-second order for Cr(VI). Further, the energy of activation for adsorption of Cr(III) is determined to be 66.82 kJ mol−1, suggesting a strong attraction between the adsorbate and the biosorbent. Application of the intraparticle diffusion model to kinetics data within the early stages of the adsorption process of Cr(III) suggests that boundary layer-retarded diffusion be valid at both solution temperatures, while no retardation be possible for Cr(VI) at the warmer temperature.
Similar content being viewed by others
Introduction
Chromium is an important metal because its compounds are widely used in industries, such as leather tanning, chrome plating, glass manufacturing, wood protection, magnetic tapes, pigments and catalysis (Weng et al. 2008; Pavithra et al. 2019). Both Cr(III) and Cr(VI) oxidation states are readily found in industrial wastewater. Although Cr(III) is less toxic than Cr(VI), high amounts of Cr(III) would result in undesirable effects on human health as well as to the environment (Zhang et al. 2019). Some health effects of chromium compounds are skin irritation and ulcer, and its chronic exposure can damage liver, kidney, and circulatory and nervous tissues (Khan and Ganai 2019). Information about the toxicity and environmental issues due to metal ions has been obtained through bioluminescence inhibition assay techniques (Abbas et al. 2018; Iqbal et al. 2019). Therefore, removal of chromium species from aquatic environments has become an utmost necessity in order to have quality life.
In this context, biosorption, which uses naturally available substances, has become an economically and environmentally friendly approach for the removal of heavy metal ions from contaminated water although this method has not been much used in large-scale industrial setting at present (Ranasinghe et al. 2018). Unlike in chemical treatment, biosorbents contain a vast number of organic compounds with many different types of functionalities which would attract heavy metal ions through complexation, in addition to other modes of mass transfer, such as ion exchange, transfer to micro- or macropores, and interparticle diffusion (Barakat 2011). Adsorption behavior of chromium on Muthurajawela peat (Priyantha et al. 2015), vesicular basalt (Alemu et al. 2018), fired brick particles (Priyantha and Seneviratne 2007), peel of jackfruit (Ranasinghe et al. 2018), palm kernel fiber, and coconut husk (Ju and Ezuma 2014) has been investigated. Organic biosorbents have more advantages, such as low cost, high efficiency, minimum chemical use, low processing conditions, low energy technology, and high metal recovery (Richards et al. 2019) as compared to synthetic materials,. Nevertheless, the major challenge to be considered is the need of a large pool of readily available biosorbent material, which would limit the application of biosorption techniques in large-scale effluent treatment facilities (Wang and Chen 2009).
Artocarpus nobilis tree is an endemic species found in the wet zone in Sri Lanka. The peel of fruit, having no economic value, is disposed of as a waste material. It has been reported that Artocarpus nobilis fruit peel demonstrates remarkable characteristics as a biosorbent for Ni(II) removal (Priyantha and Kotabewatta 2019). Generally, adsorption characteristics of biosorbents can be enhanced through surface modification which would alter surface functionalities and the surface pore structure. Modified surface thereafter would behave as a new adsorbent, showing different characteristics resembling those of the modifying agent. Common modifying agents include NaOH, HCl, CH3COOH, HNO3, EDTA, citric acid, and sodium dodecyl sulfate (SDS) (Ramana et al. 2012; Ranasinghe et al. 2018).
The main objective of this study was to investigate kinetics behavior of Cr(III) and Cr(VI) on the biosorbent prepared from the peel of Artocarpus nobilis fruits after its modification with aqueous NaOH solution through pseudo-first-order and pseudo-second-order models. Further investigation of adsorption kinetics was performed using the intraparticle diffusion model. Superior nature of the performance of the NaOH-modified biosorbent as compared to that of the unmodified biosorbent and the identification of functional groups of the modified biosorbent responsible for enhanced removal were also investigated.
Materials and methods
Materials and sample preparation
The air-dried peel of Artocarpus nobilis fruit (biosorbent) was ground well to obtain particles of diameter 0.710 mm < d < 1 mm, manually homogenized and treated with aqueous NaOH solutions of different concentrations for 1.0 h under stirred conditions followed by 24 h settling. Modified peel samples were labeled depending on the concentration of the NaOH solution used for modification (NWBF1—0.005 M NaOH; NWBF2—0.010 M NaOH; NWBF3—0.020 M NaOH; NWBF4—0.050 M NaOH; NWBF5—0.10 M NaOH; NWBF6—0.15 M NaOH). Thereafter, NaOH-modified samples were washed thoroughly with tap water followed by distilled water and air-dried for further experiments. Analytical grade K2Cr2O7 (Sigma-Aldrich) and Cr2(SO4)3.6H2O were used to prepare standard solutions in distilled water.
Instrumentation
The total chromium concentration was determined using Thermo M Series atomic absorption spectrophotometer (AAS). The methylene blue concentrations were determined using UV–visible spectrophotometer (SHIMADZU UV-1800) at 664 nm, and the surface functional groups were determined using Fourier-transform infrared/attenuated total reflectance (FTIR/ATR) spectrophotometer (Jasco 6700).
Specific surface area
First, a sample of 0.200 g of NWBF2was stirred with 10.00 mL of deionized water. Then, a sample of 0.0120 g of MB was dissolved in 100.00 mL3 of deionized water. These two solutions were allowed to mix for 2.0 h and allowed to stand overnight. An aliquot of 5.00 mL3 of the supernatant solution was withdrawn and centrifuged, and the concentration of methylene blue in the supernatant was determined. The same procedure was performed for MB of different concentrations.
The surface area of NWBF2 was calculated using the following equation:
where Ss is the surface area of NWBF2 (m2 g−1), mMB is the mass of MB adsorbed at the point of complete replacement (g), Av is the Avogadro constant (mol−1), AMB is the area covered by a MB molecule (1.3 × 10−18 m2) (Priyantha et al. 2018), ms is the mass of NWBF2 sample (g), and M is the molar mass of MB (319.87 g mol−1).
Batch adsorption studies
Batch experiments were carried out using 50.0 mL of 10 mg L−1 Cr(III) or Cr(VI) solutions. The mass of metal ions adsorbed per unit mass of the biosorbent is given by Eq. (2) (Dissanayake et al. 2016):
where \( q_{\text{e}} \) is the metal uptake (mg kg−1), Ci (mg L−1) and Cf (mg L−1) are the initial and final metal concentrations in solution, respectively, and V (L) and m (kg) are the solution volume and mass of the adsorbent, respectively (Ramana et al. 2012). The percentage of the metal ion removed (R) was calculated using Eq. (3) (Sadeek et al. 2015):
Investigation of kinetics
Kinetics studies were conducted for NWBF2 samples at pH = 5.0 for Cr(III) and at pH = 2.0 for Cr(VI). The experiments were carried out for 180-min time duration, taking samples at 1.0-min intervals within the first 30 min and then at 10-min time intervals within the next 150 min. The chromium content in each sample was determined using AAS. In this study, three different biosorbent dosages, two different adsorbate concentrations and two different temperatures were employed.
The chromium content determined at each reaction time was used to check the validity of pseudo-order kinetics models and the intraparticle diffusion model.
Results and discussion
Investigation of functional groups present in the biosorbent
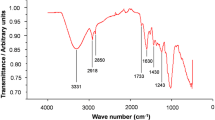
FTIR analysis performed for unmodified and modified biosorbent (NWBF 2) indicates the presence of functional groups of carboxylic acid and its derivatives, phenols, and alcohols (Fig. 1, Table 1). These observations are supported by previous reports on the same biosorbent (Priyantha and Kotabewatta 2019). Although treatment of the biosorbent with NaOH solution leads to hydrolysis of esters and ionization of carboxylic acid and phenolic groups, C=O and C–O groups remain unchanged. However, change in the chemical environment of these groups due to NaOH treatment would have caused small shifts in peak locations as observed in Fig. 1.
Optimization of the concentration of modifying agent
Variation of the extent of removal of Cr(III) and Cr(VI) by the biosorbent modified with a series of NaOH solutions is shown in Fig. 2. An increase in the concentration of NaOH solution up to 0.010 M leads to an increase in the extent of removal of Cr(III). This can be attributed to the complexation of Cr(III) with negatively charged moieties formed as a result of hydrolysis of organic functionalities during NaOH treatment. Enhancement of Cr(III) removal by different biosorbents modified with NaOH solutions has been reported supporting the observations made in this research (Bishnoi et al. 2007; Ranasinghe et al. 2018). The highest Cr(III) removal was observed when the biosorbent was treated with 0.010 M NaOH solution (NWBF2). A slight decrease in the extent of removal of Cr(III) when the modification was performed using NaOH solutions of concentrations greater that 0.010 M is probably due to denaturing of the adsorption sites of the biosorbent. The denaturing process has not become significant up to 0.15 M NaOH concentration, the highest concentration attempted in this study. In contrast to Cr(III) removal, there is a smaller increase in the extent of Cr(VI) removal with the NaOH-modified biosorbent up to 0.020 M concentration. It should be stated that the conversion of Cr(VI) to Cr(III) would occur at the acidic pH of 2.0 employed in Cr(VI) removal studies. Reduction of Cr(VI) to Cr(III) would also be possible due to certain functional groups present in natural biosorbents as reported earlier (Priyantha and Bandaranayaka 2011). Thus, Cr(VI) removal at this pH is actually the removal of Cr(III). Nevertheless, a sharp decrease in the extent of removal of Cr(VI) observed with the biosorbent treated with NaOH solutions of higher concentrations may be due to kinetics limitations because both the reduction of Cr(VI) and subsequent complexation with negatively charged surface moieties are involved in the removal process. Consequently, further studies were carried out using NWBF2 biosorbent for removal of both Cr(VI) and Cr(III).
Determination of surface area of biosorbent
The specific surface area is an important parameter of porous biosorbent surfaces. It influences the surface charge and the density of the surface sites. The use of MB provides an accurate determination of the surface area of biosorbents as it is assumed that MB molecules lie flat on the biosorbent surface due to coulombic interaction resulting in chemisorption. Equation (1) derived with the above assumption is thus used for surface area determinations. The variation of the mass of MB adsorbed versus its initial concentration shown in Fig. 3 is used to obtain the mass of MB adsorbed at the point of complete replacement (mMB), which is 478 mg g−1, and the surface area of NWBF2 is thus estimated to be 1.2 × 103 m2 g−1. This is much larger than that of many reported adsorbents, such as brick clay, rice husk, kaolinite, and Hydrilla verticillata (Bhattacharyya and Gupta 2006; Priyantha and Bandaranayaka 2011; Chathurangaa et al. 2014; Priyantha et al. 2018). More importantly, the surface area of NWBF2 is found to be larger than that of chemically modified activated carbon prepared by H3PO4 treatment of coconut shells (578 m2 g−1 to 1176 m2 g−1) and copper nitrate trihydrate treatment of carbon fiber (1147 m2 g−1) (Zhang et al. 2017; Rehman et al. 2018), demonstrating that NWBF2 shows superb adsorption properties.
Effect of solution pH on biosorption
Solution properties and the chemistry of adsorbents depend on pH, and hence, the extent of adsorption of both chromium species is influenced by the solution pH. The extent of removal versus pH investigated from the initial pH of 1.0 up to a value of the initiation of the corresponding hydroxide precipitation estimated based on the solubility product of Cr(III) from 10.0 mg L−1 solution is shown in Fig. 4.
At low pH, functional groups of the biosorbent become protonated and easily attract negatively charged adsorbate species. A decrease in the extent of Cr(III) removal and an increase in Cr(VI) removal at low solution pH can be attributed to the above fact. According to the observations shown in Fig. 4, solution pH of 2.0 is the best compromise for removal of Cr(III) and Cr(VI) species if they are both present in solution. However, for the individual removal of Cr(III), the optimum pH which shows the highest absorption is 4–6. Therefore, adsorbate solutions of Cr(III) were maintained at pH of 5.0 in subsequent experiments. At the acidic pH of 2.0, the recommended pH for Cr(VI) removal, a significant fraction of Cr(VI) would have been converted to Cr(III) as per the following reaction having a high positive standard reduction potential:
In addition to the above reaction, predominant chromium species present in the aqueous phase depends on the pH and the reduction potential according to Pourbaix diagram: The main species is H2CrO4 at pH < 1; CrO4− and Cr2O72− are mainly present between pH 2 and pH 6; CrO42− is predominant above pH 6 (Saha and Orvig 2010). Consequently, interaction of chromium species from the solution phase to solid adsorbent phase is complex having many mechanisms, such as anionic adsorption, adsorption-coupled reduction, anionic and cationic adsorption, and reduction and anionic adsorption. Further, the nature and behavior of various forms of chromium species present in wastewater could be different from those in natural water due to different physicochemical conditions of industrial effluents originating from various sources (Rakhunde et al. 2012).
Investigation of adsorption kinetics
Mass of adsorbent transferred from solution to solid adsorbent phase investigated as a function of contact time obtained in solid/solution adsorption systems is usually fitted to pseudo-order kinetics and intraparticle diffusion models. Linearized forms of pseudo-first-order and pseudo-second-order kinetics models are shown in Eqs. (4) and (5), respectively (Jacques et al. 2007):
where \( q_{e} \) and \( q_{t} \) denote the amounts of Cr(III) and Cr(VI) adsorbed per unit mass of the biosorbent (mg kg−1 dry biomass) at equilibrium and at time t, respectively, and k1 and k2 are the pseudo-first-order rate constant (min−1) and the pseudo-second-order rate constant (kg mg−1 min−1), respectively.
The amounts of Cr(III) and Cr(VI) adsorbed from individual solutions determined in batch experiments within a period of 3.0 h result in the expected variation where the system reaches equilibrium at longer times as shown in Fig. 5. The time required for establishment of adsorption equilibrium is longer for lower adsorbent dosages. Therefore, it would be more appropriate to investigate kinetics with lower adsorbent dosages because the change in the concentration of the adsorbate with respect to the saturation limit can be considered to be negligible, thereby the pseudo-order conditions can be applied with respect to the adsorbate. Consequently, adsorption data gathered during the initial 20-min period were used to check the validity of pseudo-order kinetics models. The regression coefficients (R2) determined using linearized equations of pseudo-first- and pseudo-second-order kinetics models indicate that both Cr(III) and Cr(VI) obey pseudo-first-order kinetics model for all the adsorbent dosages attempted at 27.5 °C (Fig. 6, Table 2). Thus, it can be argued that there are no distinct differences among the functionalities present in the biosorbent for attraction of chromium species. It was further determined that the order does not change with the concentration of adsorbate solution, supporting the above fact (Fig. 6, Table 2). Although the rate constant of a chemical reaction is concentration independent, it is observed to increase it with the increase in the mass of the adsorbent. This is probably due to the complex nature of the heterogeneous adsorbate/adsorbent system. Concentration-dependent rate constants have been reported in many heavy metal adsorbate–biosorbent systems (Alyuz and Veli 2009; Ali et al. 2016). However, rate constant was increased when the temperature was increased for Cr(III) adsorption system. Nevertheless, as the order of Cr(VI) adsorption changes at the warmer temperature (i.e., at 40.0 °C), the rate constants obtained for two different reaction orders cannot be compared.
Linearized pseudo-first-order kinetics models for adsorption of Cr(III) at pH 5 = 0 and Cr(VI) at pH = 2.0 on NWBF2 from 1.0 L adsorbate solution at 27.5 °C; a 10.0 mg L−1 Cr(III) on 1.00 g, b 10.0 mg L−1 Cr(III) on 2.00 g, c 10.0 mg L−1 Cr(III) on 4.00 g, d 5.0 mg L−1 Cr(III) on 1.00 g, e 10.0 mg L−1 Cr(VI) on 1.00 g, f 10.0 mg L−1 Cr(VI) on 2.00 g, g 10.0 mg L−1 Cr(VI) on 4.00 g, h 5.0 mg L−1 Cr(VI) on 1.00 g
It is found that both Cr(III) and Cr(VI) species are more effectively adsorbed on NWBF2 at warmer temperatures, indicating that the biosorption process is endothermic (Fig. 7). Further, the pseudo-order condition for biosorption of Cr(III) on NWBF2 remains the same when the solution temperature is increased from an ambient value of 27.5 °C to 40.0 °C, indicating that the mechanism of biosorption of Cr(III) is not changed at warmer temperatures (Fig. 8). The rate constants determined for biosorption of Cr(III) on NWBF2 at the two temperatures lead to the energy of activation of 66.82 kJ mol−1, determined with the aid of the linearized form of the Arrhenius equation, qualifying that the biosorption of Cr(III) follows chemisorption. Adsorption of heavy metals on different adsorbents has been reported to follow chemisorption (Kumar and Acharya 2011; Cantu et al. 2014). On the other hand, biosorption of Cr(VI) on NWBF2 at 40.0 °C obeys pseudo-second-order kinetics, based on regression analysis of the linearized equation, indicating that there are two distinct types of interactions (Fig. 8). It can thus be argued that carboxylic acid and phenolic acid moieties would function differently toward biosorption of Cr(VI) at warmer temperatures. As the mechanism of biosorption of Cr(VI) is changed when the temperature is increased, it would not be appropriate to use the Arrhenius equation in determining the energy of activation.
Intraparticle diffusion model
This model introduced by Weber and Morris states that solute uptake on adsorbent is proportional to the square root of time. The mathematical relationship of this model is given in Eq. (6):
where \( q_{t} \) is the amount adsorbed at time t, \( k_{p} \) (mg kg−1 min−1/2) is the intraparticle diffusion rate constant, and C (mg kg−1) is the boundary layer thickness (Wu et al. 2009).
The plots of Eq. (6) for adsorption of Cr(III) and Cr(VI) at two solution temperatures are shown in Fig. 9. Accordingly, the boundary layer thickness values are estimated to be − 0.073 and − 0.025 for Cr(III) at solution temperatures of 27.5 °C and 40.0 °C, respectively (Table 3). The corresponding values for Cr(VI) are − 0.088 and + 1.404, respectively. The negative values for C are indicative of boundary layer-retarded diffusion (Wu et al. 2009), which should increase when the solution temperature is increased as the rate of diffusion is facilitated by temperature increments. This is clear in both cases, Cr(III) and Cr(VI), according to the values reported in Table 3. In the adsorption of Cr(VI), a significant increase in the value of C is observed, in comparison with the smaller increase in relation to Cr(III). This can be attributed to the change in reaction mechanism for adsorption of Cr(VI) from pseudo-first order to pseudo-second order when the temperature is increased from 27.5 to 40.0 °C.
Conclusions
Modification of the peel of Artocarpus nobilis fruit through treatment with aqueous NaOH solution leads to the ionization of carboxylic and phenolic acid functional groups and cleavage of ester linkages, thereby promoting the interaction of the above biosorbent and Cr(III) species. On the other hand, Cr(VI) in solution would undergo reduction in extreme acidic medium forming Cr(III) and subsequently attracted to negatively charged biosorbent surface. In addition to the above mode of mass transfer from solution phase to solid biosorbent phase, enhancement of the surface area of the biosorbent through NaOH treatment would make the removal process more efficient. The interaction of both Cr(III) and Cr(VI) ions with NaOH-modified biosorbent is determined to follow pseudo-first-order kinetics with respect to the biosorbent at the ambient temperature of 27.5 °C when the concentration of the adsorbate species is kept relatively unchanged within the early stages of adsorption. An increase in solution temperature up to 40.0 °C does not change the order of the adsorption reaction of Cr(III) although Cr(VI) species follow pseudo-second-order kinetics, suggesting the involvement of two distinct types of functional groups of the biosorbent. Intraparticle diffusion model provides further support for the above observations, in which the extent of boundary layer-retarded diffusion is not much changed for Cr(III) adsorption when the solution temperature is changed from 27.5 to 40.0 °C, while there is a significant increase for Cr(VI) adsorption.
Abbreviations
- NWBF1:
-
0.005 M NaOH-treated peel of Artocarpus nobilis fruit
- NWBF2:
-
0.010 M NaOH-treated peel of Artocarpus nobilis fruit
- NWBF3:
-
0.020 M NaOH-treated peel of Artocarpus nobilis fruit
- NWBF4:
-
0.050 M NaOH-treated peel of Artocarpus nobilis fruit
- NWBF5:
-
0.100 M NaOH-treated peel of Artocarpus nobilis fruit
- NWBF6:
-
0.150 M NaOH-treated peel of Artocarpus nobilis fruit
- MB:
-
Methylene blue
References
Abbas MI, Adil M, Ehtisham-Ul-Haque S, Munir B, Yameen M, Ghaffar A, Shar GA, Asif Tahir M, Iqbal M (2018) Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: a review. Sci Total Environ 626:1295–1309
Alemu A, Lemma B, Gabbiye N, Tadele M, Teferi M (2018) Removal of chromium (VI) from aqueous solution using vesicular basalt: a potential low cost wastewater treatment system. Heliyon 4:2405–8440
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332
Alyuz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377
Bhattacharyya KG, Gupta SS (2006) Adsorption of chromium (VI) from water by clays. Ind Eng Chem 45:7232–7240
Bishnoi NR, Kumar R, Kumar S, Rani S (2007) Biosorption of Cr(III) from aqueous solution using algal biomass Spirogyra Spp. J Hazard Mater 145(1–2):142–147
Cantu Y, Remes A, Reyna A, Martinez D, Villarreal J, Ramos H, Samantha Trevino S, Tamez C, Martinez A, Eubanks T, Parsons JG (2014) Thermodynamics, kinetic sand activation energy studies of the sorption of chromium(III) and chromium(VI) to a Mn3O4 nanomaterial. Chem Eng J 254:374–383
Chathurangaa PKD, Dissanayake DMERA, Priyantha N, Iqbal SS, Iqbal MCM (2014) Biosorption and desorption of lead (II) by Hydrilla verticillata. Bioremediat J 18:37–41
Dissanayake DMREA, WijesingheWMKEH Iqbal SS, Priyantha N, Iqbal MCM (2016) Isotherm and kinetic study on Ni(II) and Pb(II) biosorption by the fern Asplenium nidus L. Ecol Eng 88:237–241
Iqbal M, Abbas M, Nisar J, Nazir A, Qamar A (2019) Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: a review. Chem Int 5(1):1–80
Jacques RA, Lima EC, Dias SLP, Mazzocato AC, Pavan FA (2007) Yellow passion-fruit shell as biosorbent to remove Cr(III) and Pb(II) from aqueous solution. Sep Purif Technol 57(1):193–198
Ju O, Ezuma I (2014) Adsorption studies of heavy metals by low-cost adsorbents. J Appl Sci Environ Manag 18(3):443–448
Khan AM, Ganai SA (2019) Removal and recovery of heavy metal ions using natural adsorbents. Modern Age Water Problems 251–260
Kumar U, Acharya J (2011) Thermodynamics of the sorption of Pb(II) from aqueous solution on NCRH. Int J Chem Eng Appl 2(6):416–420
Pavithra KG, Jaikumar Kumar VPS, Sundarrajan PS (2019) A review on cleaner strategies for chromium industrial wastewater: present research and future perspective. J Clean Prod 228:580–593
Priyantha N, Bandaranayaka A (2011) Investigation of kinetics of Cr(VI)– fired brick clay interaction. J Hazard Mater 188:193–197
Priyantha N, Kotabewatta PA (2019) Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni (II) sorption under static and dynamic conditions. Appl Water Sci 9:1–10
Priyantha N, Navaratne AN, Kulasooriya TPK (2018) Kulasooriya TPK (2018) Investigation on adsorption kinetics of heavy metals by rice husk. J Natl Sci Found Sri Lanka 46(2):125–141
Priyantha N, Seneviratne C (2007) Adsorption of chromium (III) on fired brick particles. Proc Peradeniya Univ Res Sessions Sri Lanka 12:296–297
Priyantha N, Lim LBL, Wickramasooriya S (2015) Adsorption behaviour of Cr(VI) by Muthurajawela peat. Desalin Water Treat 57:1–9
Rakhunde R, Deshpande L, Juneja HD (2012) Chemical speciation of chromium in water: a review. Environ Sci Technol 42(7):776–810
Ramana DKV, Reddy DHK, Kumar BN, Harinath Y, Seshaiah K (2012) Removal of Nickel from Aqueous Solutions by citric acid modified Ceiba pentandra hulls: equilibrium and kinetic studies. Can J Chem Eng 90:111–119
Ranasinghe RH, Navaratne AN, Priyantha N (2018) Enhancement of adsorption characteristics of Cr(III)and Ni(II) by surface modification of jackfruit peel biosorbent. Environ Chem Eng 6:5670–5682
Rehman A, Baek JW, Rene ER, Sergienko N, Behera SK, Hung-Suck Park H-S (2018) Effect of process parameters influencing the chemical modification of activated carbon fiber for carbon dioxide removal. Process Saf Environ Prot 118:384–396
Richards S, Dawson J, Stutter M (2019) The potential use of natural vs commercial biosorbent material to remediate stream waters by removing heavy metal contaminants. J Environ Manag 231:275–281
Sadeek SA, Negm NA, Hefni HHH, Wahab MMA (2015) Metal adsorption by agricultural biosorbents: adsorption isotherm, kinetic and biosorbents chemical structures. Int J Biol Macromol 81:400–409
Saha B, Orvig C (2010) Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord Chem Rev 254(23–24):2959–2972
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2):195–226
Weng CH, Sharma YC, Chu SH (2008) Adsorption of Cr(VI) from aqueous solutions by spent activated clay. J Hazard Mater 155(1–2):65–75
Wu F, Tseng R, Juang R (2009) Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem Eng J 153:1–8
Zhang M, Li Y, Si H, Wang B, Song T (2017) Preparation and electrochemical performance of coconut shell activated carbon produced by the H3PO4 activation with rapid cooling method. Int J Electrochem Sci 12:7844–7852
Zhang Q, Song Y, Amor K, Huang WE, Porcelli D, Thompson I (2019) Monitoring Cr toxicity and remediation processes—combining a whole-cell bioreporter and Cr isotope techniques. Water Res 153:295–303
Acknowledgements
This study was funded by World Bank (Grant Number: 6026-LK/8743-LK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samaraweera, A.P.G.M.V., Priyantha, N., Gunathilake, W.S.S. et al. Biosorption of Cr(III) and Cr(VI) species on NaOH-modified peel of Artocarpus nobilis fruit. 1. Investigation of kinetics. Appl Water Sci 10, 115 (2020). https://doi.org/10.1007/s13201-020-01187-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01187-2