Abstract
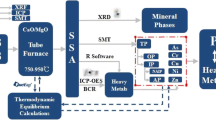
Phosphorus (P) is an essential and limited resource. Incineration sewage sludge ash (ISSA) contains a high amount of P, which can be recovered using chemical leaching methods. However, the recovery ratio depends on the speciation of P and the leaching conditions. In this study, hydrochloric acid was used as a leaching agent, and the effects of the hydrochloric acid concentration, leaching time, temperature, and liquid–solid ratio on the P leaching ratio were investigated. Furthermore, the co-leaching of macro-metals Ca, Al, Fe, and Mg was analyzed. The results showed that P leached rapidly within 30 min, where the leaching rate reached more than 80% and then gradually stabilized. The leaching concentrations of Ca and Mg had a significant correlation (correlation coefficient r2 > 0.90), and both were leached completely. Al and P had similar leaching patterns, where the leaching rates increased initially and then decreased with time at 0.2 mol/L HCl. According to X-ray diffraction analysis and Rietveld refinement, the P in ISSA was mainly present in the forms of Mg3Ca3(PO4)4 and AlPO4. When leached using 0.2 mol/L HCl at 55 °C with a liquid-to-solid ratio of 20 L/kg, the AlPO4 and Fe3(H2O)3(PO4)2 in ISSA dissolved initially and then precipitated on the surface of the solid phase, thereby impeding further P leaching.







Similar content being viewed by others
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Petzet S, Peplinski B, Cornel P. On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res. 2012;46(12):3769–80. https://doi.org/10.1016/j.watres.2012.03.068.
Jasinski SM. Minerals Yearbook: phosphate rock. Phosphate rock. U.S.Geological Survey; 2016.
Cordell D, Drangert J-O, White S. The story of phosphorus: Global food security and food for thought. Glob Environ Change. 2009;19(2):292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009.
Xue YG, Wu FF, Liu XJ, et al. A review on phosphorus recovery technology and Its application in the sewage sludge recycling. Environ Sci Technol. 2014;37(S2):247–51.
Smil V. Phosphorus in the environment: natural flows and human interferences. Annu Rev Energy Env. 2000;25(1):53–88. https://doi.org/10.1146/annurev.energy.25.1.53.
Franz M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manage. 2008;28(10):1809–18. https://doi.org/10.1016/j.wasman.2007.08.011.
Cornel P, Schaum C. Phosphorus recovery from wastewater: needs, technologies and costs. Water Sci Technol. 2009;59(6):1069–76. https://doi.org/10.2166/wst.2009.045.
Lim BH, Kim D-J. Selective acidic elution of Ca from sewage sludge ash for phosphorus recovery under pH control. J Ind Eng Chem. 2017;46:62–7. https://doi.org/10.1016/j.jiec.2016.10.016.
Herzel H, Kruger O, Hermann L, et al. Sewage sludge ash–A promising secondary phosphorus source for fertilizer production. Sci Total Environ. 2016;542(Pt B):1136–43. https://doi.org/10.1016/j.scitotenv.2015.08.059.
Chen T, Yan B. Fixation and partitioning of heavy metals in slag after incineration of sewage sludge. Waste Manage. 2012;32(5):957–64. https://doi.org/10.1016/j.wasman.2011.12.003.
Medici F, Piga L, Rinaldi G. Behaviour of polyaminophenolic additives in the granulation of lime and fly-ash. Waste Manage. 2000;20(7):491–8. https://doi.org/10.1016/S0956-053X(00)00030-1.
Pettersson A, Åmand L-E, Steenari B-M. Leaching of ashes from co-combustion of sewage sludge and wood—Part I: Recovery of phosphorus. Biomass Bioenerg. 2008;32(3):224–35. https://doi.org/10.1016/j.biombioe.2007.09.016.
Adam C, Peplinski B, Michaelis M, et al. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manage. 2009;29(3):1122–8. https://doi.org/10.1016/j.wasman.2008.09.011.
Lee M, Kim D-J. Identification of phosphorus forms in sewage sludge ash during acid pre-treatment for phosphorus recovery by chemical fractionation and spectroscopy. J Ind Eng Chem. 2017;51:64–70. https://doi.org/10.1016/j.jiec.2017.02.013.
Fang L, Li JS, Guo MZ, et al. Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere. 2018;193:278–87. https://doi.org/10.1016/j.chemosphere.2017.11.023.
Kalmykova Y, Karlfeldt FK. Phosphorus recovery from municipal solid waste incineration fly ash. Waste Manage. 2013;33(6):1403–10. https://doi.org/10.1016/j.wasman.2013.01.040.
Li J-S, Chen Z, Wang Q-M, et al. Change in re-use value of incinerated sewage sludge ash due to chemical extraction of phosphorus. Waste Manage. 2018;74:404–12. https://doi.org/10.1016/j.wasman.2018.01.007.
Biswas BK, Inoue K, Harada H, et al. Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J Environ Sci. 2009;21(12):1753–60. https://doi.org/10.1016/S1001-0742(08)62484-5.
Ottosen LM, Kirkelund GM, Jensen PE. Extracting phosphorous from incinerated sewage sludge ash rich in iron or aluminum. Chemosphere. 2013;91(7):963–9. https://doi.org/10.1016/j.chemosphere.2013.01.101.
Falayi T. Alkaline recovery of phosphorous from sewage sludge and stabilisation of sewage sludge residue. Waste Manage. 2019;84:166–72. https://doi.org/10.1016/j.wasman.2018.11.041.
Meng X, Liu X, Huang Q, et al. Recovery of phosphate as struvite from low-temperature combustion sewage sludge ash (LTCA) by cation exchange. Waste Manage. 2019;90:84–93. https://doi.org/10.1016/j.wasman.2019.04.045.
Wang Q, Li J-S, Tang P, et al. Sustainable reclamation of phosphorus from incinerated sewage sludge ash as value-added struvite by chemical extraction, purification and crystallization. J Clean Prod. 2018;181:717–25. https://doi.org/10.1016/j.jclepro.2018.01.254.
Xu H, He P, Gu W, et al. Recovery of phosphorus as struvite from sewage sludge ash. J Environ Sci. 2012;24(8):1533–8. https://doi.org/10.1016/S1001-0742(11)60969-8.
Donatello S, Tong D, Cheeseman CR. Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manage. 2010;30(8–9):1634–42. https://doi.org/10.1016/j.wasman.2010.04.009.
Li R, Zhang Z, Li Y, et al. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere. 2015;141:57–61. https://doi.org/10.1016/j.chemosphere.2015.05.094.
Shiba NC, Ntuli F. Extraction and precipitation of phosphorus from sewage sludge. Waste Manage. 2017;60:191–200. https://doi.org/10.1016/j.wasman.2016.07.031.
Yang F, Chen J, Yang M, et al. Phosphorus recovery from sewage sludge via incineration with chlorine-based additives. Waste Manage. 2019;95:644–51. https://doi.org/10.1016/j.wasman.2019.06.029.
Vogel C, Adam C. Heavy metal removal from sewage sludge ash by thermochemical treatment with gaseous hydrochloric acid. Environ Sci Technol. 2011;45(17):7445–500. https://doi.org/10.1021/es2007319.
Krüger O, Adam C. Phosphorus in recycling fertilizers - analytical challenges. Environ Res. 2017;155:353–8. https://doi.org/10.1016/j.envres.2017.02.034.
Winburn R, Grier D, Mccarthy GJ, et al. Rietveld quantitative X-ray diffraction analysis of NIST fly ash standard reference materials. Powder Diffr. 2000;15(3):163–72. https://doi.org/10.1017/S0885715600011015.
Gualtieri A. Modal analysis of piroclastic rocks by combined rietveld and RIR methods. Powder Diffr. 1996;11(2):97–106. https://doi.org/10.1017/S0885715600009052.
(Mep) MOEP. Solid waste-determination of 22 metal elements—inductively couples plasma optical emission spectrometry. Beijing: China Environmental Science Press; 2016.
Gualtieri ML, Prudenziati M, Gualtieri AF. Quantitative determination of the amorphous phase in plasma sprayed alumina coatings using the Rietveld method. Surf Coat Technol. 2006;201(6):2984–9. https://doi.org/10.1016/j.surfcoat.2006.06.009.
Kim W, Zhang Q, Saito F. Mechanochemical synthesis of hydroxyapatite from Ca(OH)2–P2O5 and CaO-Ca(OH)2–P2O5 mixtures. J Mater Sci. 2000;35(21):5401–5. https://doi.org/10.1023/a:1004859231795.
Hoffmann G, Schingnitz D, Bilitewski B. Comparing different methods of analysing sewage sludge, dewatered sewage sludge and sewage sludge ash. Desalination. 2010;250(1):399–403. https://doi.org/10.1016/j.desal.2009.09.064.
Budhathoki R, Väisänen A, Lahtinen M. Selective recovery of phosphorus as AlPO4 from silicon-free CFB-derived fly ash leachate. Hydrometallurgy. 2018;178:30–6. https://doi.org/10.1016/j.hydromet.2018.03.025.
Li X, Xing P, Du X, et al. Influencing factors and kinetics analysis on the leaching of iron from boron carbide waste-scrap with ultrasound-assisted method. Ultrason Sonochem. 2017;38:84–91. https://doi.org/10.1016/j.ultsonch.2017.02.037.
Maccarthy J, Nosrati A, Skinner W, et al. Acid leaching and rheological behaviour of a siliceous goethitic nickel laterite ore: Influence of particle size and temperature. Miner Eng. 2015;77:52–63. https://doi.org/10.1016/j.mineng.2014.12.031.
Hosseini SA, Raygan S, Rezaei A, et al. Leaching of nickel from a secondary source by sulfuric acid. J Environ Chem Eng. 2017;5(4):3922–9. https://doi.org/10.1016/j.jece.2017.07.059.
Fang L, Li J-S, Donatello S, et al. Recovery of phosphorus from incinerated sewage sludge ash by combined two-step extraction and selective precipitation. Chem Eng J. 2018;348:74–83. https://doi.org/10.1016/j.cej.2018.04.201.
Liu DJ, Zhong BH, Zhang YX. Liquid and solid phase reaction kinetics of phosphoric acid complex system (II) experimental study on digestion kinetics of phosphate ore particle system. CIESC J. 2001;01:28–34.
Papamichael EM, Economou ED, Vaimakis TC. Dissolution of the carbonate minerals of phosphate ores: catalysis by carbonic anhydrase II, from bovine erythrocytes, in acid solutions. J Colloid Interface Sci. 2002;251(1):143–50. https://doi.org/10.1006/jcis.2002.8366.
Economou ED, Vaimakis TC, Papamichael EM. The kinetics of dissolution of the carbonate minerals of phosphate ores using dilute acetic acid solutions: the case of pH range from 3.96 to 6.40. J Colloid Interface Sci. 2002;245(1):133–41. https://doi.org/10.1006/jcis.2001.7931.
Li M, Wei C, Qiu S, et al. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy. 2010;104(2):193–200. https://doi.org/10.1016/j.hydromet.2010.06.001.
Dickinson CF, Heal GR. Solid-liquid diffusion controlled rate equations. Thermochim Acta. 1999;340–341:89–103. https://doi.org/10.1016/S0040-6031(99)00256-7.
Tanada S, Kabayama M, Kawasaki N, et al. Removal of phosphate by aluminum oxide hydroxide. J Colloid Interface Sci. 2003;257(1):135–40. https://doi.org/10.1016/S0021-9797(02)00008-5.
Acknowledgements
This work was supported by the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07202005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, P., Zhang, X., Lü, F. et al. Leaching behavior of phosphorous compounds from sewage sludge ash based on quantitative X-ray diffraction analysis. Waste Dispos. Sustain. Energy 2, 113–125 (2020). https://doi.org/10.1007/s42768-020-00037-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-020-00037-w