Abstract
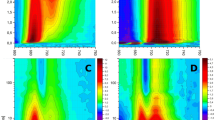
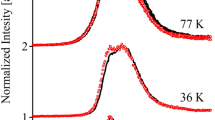
Light-harvesting complex II (LHCII) is the major antenna complex in higher plants and green algae. It has been suggested that a major part of the excited state energy dissipation in the so-called “non-photochemical quenching” (NPQ) is located in this antenna complex. We have performed an ultrafast kinetics study of the low-energy fluorescent states related to quenching in LHCII in both aggregated and the crystalline form. In both sample types the chlorophyll (Chl) excited states of LHCII are strongly quenched in a similar fashion. Quenching is accompanied by the appearance of new far-red (FR) fluorescence bands from energetically low-lying Chl excited states. The kinetics of quenching, its temperature dependence down to 4 K, and the properties of the FR-emitting states are very similar both in LHCII aggregates and in the crystal. No such FR-emitting states are found in unquenched trimeric LHCII. We conclude that these states represent weakly emitting Chl–Chl charge-transfer (CT) states, whose formation is part of the quenching process. Quantum chemical calculations of the lowest energy exciton and CT states, explicitly including the coupling to the specific protein environment, provide detailed insight into the chemical nature of the CT states and the mechanism of CT quenching. The experimental data combined with the results of the calculations strongly suggest that the quenching mechanism consists of a sequence of two proton-coupled electron transfer steps involving the three quenching center Chls 610/611/612. The FR-emitting CT states are reaction intermediates in this sequence. The polarity-controlled internal reprotonation of the E175/K179 aa pair is suggested as the switch controlling quenching. A unified model is proposed that is able to explain all known conditions of quenching or non-quenching of LHCII, depending on the environment without invoking any major conformational changes of the protein.







Similar content being viewed by others
Notes
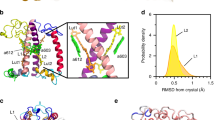
The crystal structure of LHCII (Standfuss et al. 2005) as deposited in file ID 2BHW in the protein database has been used as structural basis.
We note here that the three-state model used by Chmeliov et al. (two fluorescing components, plus one non-fluorescent “dark state”) (Chmeliov et al. 2016) is kinetically indistinguishable in fluorescence data from a simpler two-state model with two fluorescent components since presence or absence of the “dark state” has no influence on the fluorescence kinetics. The proposal of the “dark state” in the kinetic model is thus an unproven assumption lacking experimental evidence.
Due to the pronounced CT state relaxation at higher temperatures this system does not show the temperature dependence of reaction rates expected for a normal Boltzmann equilibrium.
The authors (Gelzinis et al. 2018) claim that they have tested our Chl–Chl CT state kinetic model (Miloslavina et al. 2008; Müller et al. 2010) and found it to be inconsistent with their fluorescence kinetic data on LHCII aggregates. We note that their interpretation is a gross misinterpretation of our CT state model, since they modeled an energy transfer process as populating the “red state”. In contrast in our Chl–Chl CT state kinetic model an electron transfer process from the excited LHCII state to the Chl–Chl CT state, residing in the same LHCII monomer as the formed CT state, populates the “red state”. state. Thus the interpretation used in their modeling (Chmeliov et al. 2016; Gelzinis et al. 2019) has no resemblance to and deviates fundamentally from our proposed kinetic model.
Abbreviations
- LHCII:
-
Major light-harvesting complex of photosystem II
- PSII:
-
Photosystem II
- RC:
-
Reaction center
- Chl:
-
Chlorophyll
- Car:
-
Carotenoid
- Lut:
-
Lutein
- Vx:
-
Violaxanthin
- Zx:
-
Zeaxanthin
- Nx:
-
Neoxanthin
- DAES:
-
Decay-associated emission spectrum
- r.t:
-
Room temperature
- NPQ:
-
Non-photochemical quenching
- qE:
-
Energy-dependent quenching
- CT (state):
-
Charge-transfer (state)
- RP:
-
Radical pair
- FR:
-
Far-red
- HL:
-
High light
References
Akhtar P, Dorogi M, Pawlak K, Kovacs L, Bota A, Kiss T, Garab G, Lambrev PH (2015) Pigment interactions in light-harvesting complex II in different molecular environments. J Biol Chem 290:4877–4886
Akhtar P, Görföl F, Garab G, Lambrev PH (2019a) Dependence of chlorophyll fluorescence quenching on the lipid-to-protein ratio in reconstituted light-harvesting complex II membranes containing lipid labels. Chem Phys 522:242–248
Akhtar P, Lindorfer D, Lingvay M, Pawlak K, Zsiros O, Siligardi G, Javorfi T, Dorogi M, Ughy B, Garab G, Renger T, Lambrev PH (2019b) Anisotropic circular dichroism of light-harvesting complex ii in oriented lipid bilayers: theory meets experiment. J Phys Chem B 123:1090–1098
Amarie S, Arefe K, Starcke JH, Dreuw A, Wachtveitl J (2008) Identification of an additional low-lying excited state of carotenoid radical cations. J Phys Chem B 112:14011–14017
Amarie S, Standfuss J, Barros T, Kühlbrandt W, Dreuw A, Wachtveitl J (2007) Carotenoid radical cations as a probe for the molecular mechanism of nonphotochemical quenching in oxygenic photosynthesis. J Phys Chem B 111:3481–3487
Amarie S, Wilk L, Barros T, Kühlbrandt W, Dreuw A, Wachtveitl J (2009) Properties of zeaxanthin and its radical cation bound to the minor light-harvesting complexes CP24, CP26 and CP29. Biochim Biophys Acta-Bioenergetics 1787:747–752
Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283:3550–3558
Balevicius V Jr, Fox KF, Bricker WP, Jurinovich S, Prandi IG, Mennucci B, Duffy CDP (2017) Fine control of chlorophyll-carotenoid interactions defines the functionality of light-harvesting proteins in plants. Sci Rep 7:13956
Barros T, Royant A, Standfuss J, Dreuw A, Kühlbrandt W (2009) Crystal structure of plant light-harvesting complex shows the active, energy-transmitting state. EMBO J 28:298–306
Beechem JM, Brand L (1985) Time-resolved fluorescence of proteins. Ann Rev Biochem 54:43–71
Bennett DIG, Amarnath K, Park S, Steen CJ, Morris JM, Fleming GR (2019) Models and mechanisms of the rapidly reversible regulation of photosynthetic light harvesting. Open Biol 9:190043
Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, Dall'Osto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284:15255–15266
Bixon M, Jortner J (1969) Electronic relaxation in large molecules. J Chem Phys 50:4061–4070
Bixon M, Jortner J, Cortes J, Heitele H, Michel-Beyerle ME (1994) Energy gap law for nonradiative and radiative charge transfer in isolated and in solvated supermolecules. J Phys Chem 98:7289–7729
Bixon M, Michel-Beyerle ME, Jortner J (1988) Formation dynamics, decay kinetics, and singlet-triplet splitting of the (bacteriochlorophyll dimer)+ (bacteriopheophytin)- radical pair in bacterial photosynthesis. Isr J Chem 28:155–168
Bode S, Quentmeier CC, Liao PN, Barros T, Walla PJ (2008) Xanthophyll-cycle dependence of the energy transfer between carotenoid dark states and chlorophylls in NPQ mutants of living plants and in LHC II. Chem Phys Lett 450:379–385
Bode S, Quentmeier CC, Liao PN, Hafi N, Barros T, Wilk L, Bittner F, Walla PJ (2009) On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc Natl Acad Sci USA 106:12311–12316
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Chmeliov J, Bricker WP, Lo C, Jouin E, Valkunas L, Ruban AV, Duffy CD (2015) An 'all pigment' model of excitation quenching in LHCII. Phys Chem Chem Phys 17:15857–15867
Chmeliov J, Gelzinis A, Songaila E, Augulis R, Duffy CD, Ruban AV, Valkunas L (2016) The nature of self-regulation in photosynthetic light-harvesting antenna. Nat Plants 2:16045
Crisafi E, Krishnan M, Pandit A (2018) Time-resolved fluorescence analysis of LHCII in the presence of PsbS at neutral and low pH. bioRxiv 456046
Croce R, Chojnicka A, Morosinotto T, Ihalainen JA, van Mourik F, Dekker JP, Bassi R, van Grondelle R (2007) The low-energy forms of photosystem I light-harvesting complexes: Spectroscopic properties and pigment-pigment interaction characteristics. Biophys J 93:2418–2428
Croce R, Morosinotto T, Bassi R (2006) LHCI: The antenna complex of photosystem I in plants and green algae. In: Golbeck JH (ed) Photosytem I: The Light-Driven Plastocyanin: Ferredoxin Oxidoreductase. Springer, Dordrecht, pp 119–137
Croce R, Morosinotto T, Castelletti S, Breton J, Bassi R (2002) The Lhca antenna complexes of higher plants photosystem I. Biochim Biophys Acta 1556:29–40
Cupellini L, Calvani D, Jacquemin D, Mennucci B (2020) Charge transfer from the carotenoid can quench chlorophyll excitation in antenna complexes of plants. Nature Communications 11:662
Cupellini L, Corbella M, Mennucci B, Curutchet C (2019) Electronic energy transfer in biomacromolecules. Wiley Interdisciplinary Reviews: Computational Molecular Science 9:e1392
Dall'Osto L, Cazzaniga S, Bressan M, Palecek D, Zidek K, Niyogi KK, Fleming GR, Zigmantas D, Bassi R (2017) Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nat Plants 3:17033
Dapprich S, Komarómi I, Byun KS, Morokuma K, Frisch MJ (1999) A new ONIOM implementation in Gaussian98. Part I. the calculation of energies, gradients, vibrational frequencies and electric field derivatives. J Mol Struct 461–462:1–21
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Dreuw A, Fleming GR, Head-Gordon M (2003) Chlorophyll fluorescence quenching by xanthophylls. Phys Chem Chem Phys 5:3247–3256
Duffy CD, Ruban AV (2015) Dissipative pathways in the photosystem-II antenna in plants. J Photochem Photobiol B 152:215–226
Fox KF, Balevicius V, Chmeliov J, Valkunas L, Ruban AV, Duffy CDP (2017) The carotenoid pathway: what is important for excitation quenching in plant antenna complexes? Phys Chem Chem Phys 19:22957–22968
Fox KF, Unlu C, Balevicius V Jr, Ramdour BN, Kern C, Pan X, Li M, van Amerongen H, Duffy CDP (2018) A possible molecular basis for photoprotection in the minor antenna proteins of plants. Biochim Biophys Acta 1859:471–481
Frank HA, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski MR (1994) Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth Res 41:389–395
Frese RN, Palacios MA, Azzizi A, van Stokkum IHM, Kruip J, Rögner M, Karapetyan NV, Schlodder E, van Grondelle R, Dekker JP (2002) Electric field effects on red chlorophylls, beta-carotenes and P700 in cyanobacterial Photosystem I complexes. Biochim Biophys Acta 1554:180–191
Garab G, Faludi-Daniel A, Sutherland JC, Hind G (1988) Macroorganization of chlorophyll a/b light-harvesting complex in thylakoids and aggregates: Information from circular differential scattering. Biochemistry 27:2425–2430
Gatzen G, Müller MG, Griebenow K, Holzwarth AR (1996) Primary processes and structure of the photosystem II reaction center: 3. Kinetic analysis of picosecond energy transfer and charge separation processes in the D1-D2-cyt-b559 complex measured by time-resolved fluorescence. J Phys Chem 100:7269–7278
Gelzinis A, Augulis R, Butkus V, Robert B, Valkunas L (2019) Two-dimensional spectroscopy for non-specialists. Biochim Biophys Acta Bioenerg 1860:271–285
Gelzinis A, Chmeliov J, Ruban AV, Valkunas L (2018) Can red-emitting state be responsible for fluorescence quenching in LHCII aggregates? Photosynth Res 135:275–284
Gibasiewicz K, Croce R, Morosinotto T, Ihalainen JA, van Stokkum IHM, Dekker JP, Bassi R, van Grondelle R (2005) Excitation energy transfer pathways in Lhca4. Biophys J 88:1959–1969
Grimme S (1996) Density functional calculations with configuration interaction for the excited states of molecules. Chem Phys Lett 259:128–137
Guo Z, Woodbury NW, Pan J, Lin S (2012) Protein dielectric environment modulates the electron-transfer pathway in photosynthetic reaction centers. Biophys J 103:1979–1988
Hammes-Schiffer S (2009) Theory of proton-coupled electron transfer in energy conversion processes. Acc Chem Res 42:1881–1889
Hammes-Schiffer S, Hatcher E, Ishikita H, Skone JH, Soudackov AV (2008) Theoretical studies of proton-coupled electron transfer: models and concepts relevant to bioenergetics. Coord Chem Rev 252:384–394
Hayes JM, Matsuzaki S, Rätsep M, Small GJ (2000) Red chlorophyll a antenna states of photosystem I of the cyanobacterium Synechocystis sp PCC 6803. J Phys Chem B 104:5625–5633
Heitele H, Finckh P, Weeren S, Pöllinger F, Michel-Beyerle ME (1989) Solvent polarity effects of intramolecular electron transfer. 1. Energetic aspects J Phys Chem 93:5173–5179
Heitele H, Michel-Beyerle ME (1987) The influence of dielectric relaxation on intramolecular electron transfer. Chem Phys Lett 138:237–243
Heitele H, Pöllinger F, Weeren S, Michel-Beyerle ME (1990) Influence of solvent polarity on intramolecular electron transfer: a consistency test of free energies of reaction and solvent reorganization with experimental rates. Chem Phys 143:325–332
Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Holzwarth AR (1986) Excited state kinetics of chlorophyll antenna pigments. In: Staehelin LA, Arntzen CJ (eds) Encyclopedia of plant physiology: photosynthesis III new serie. 19. Springer, Berlin, pp 299–309
Holzwarth AR (1988) Time resolved chlorophyll fluorescence:. what kind of information on photosynthetic systems does it provide? In: Lichtenthaler HK (ed) Applications of Chlorophyll Fluorescence. Kluwer Academic, Dordrecht, pp 21–31
Holzwarth AR (1991) Excited-state kinetics in chlorophyll systems and its relationship to the functional organization of the photosystems. In: Scheer H (ed) Chlorophylls. CRC Press, Boca Raton, pp 1125–1151
Holzwarth AR (1995) Time-resolved fluorescence spectroscopy. In: Sauer K (ed) Methods in Enzymology, vol 246. Academic Press, San Diego, pp 334–362
Holzwarth AR (1996) Data analysis of time-resolved measurements. In: Amesz J, Hoff AJ (eds) Biophysical Techniques in Photosynthesis. Kluwer Academic Publishers, Dordrecht, Advances in Photosynthesis Research, pp 75–92
Holzwarth AR, Jahns P (2014) Non-photochemical quenching mechanisms in intact organisms as derived from ultrafast-fluorescence kinetic studies. In: Demmig-Adams B, Garab G, Adams WWI, Govindjee (eds) Non-photochemical quenching and thermal energy dissipation in plants, algae and cyanobacteria. Springer, Dordrecht, pp 129–156
Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P (2009) Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem Phys Lett 483:262–267
Holzwarth AR, Müller MG (1996) Energetics and kinetics of radical pairs in reaction centers from Rhodobacter sphaeroides. A femtosecond transient absorption study Biochemistry 35:11820–11831
Holzwarth AR, Müller MG, Reus M, Nowaczyk M, Sander J, Rögner M (2006) Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA 103:6895–6900
Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ (1991) Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett 292:1–4
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Horton P, Wentworth M, Ruban AV (2005) Control of the light harvesting function of chloroplast membranes: The LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579:4201–4206
Hughes JL, Prince BJ, Arsköld SP, Smith PJ, Pace RJ, Riesen H, Krausz E (2004) The native reaction centre of photosystem II: a new paradigm for P680. Aust J Chem 57:1179–1183
Hughes JL, Smith P, Pace R, Krausz E (2006a) Charge separation in photosystem II core complexes induced by 690–730 nm excitation at 1.7K. Biochim Biophys Acta 1757:841–851
Hughes JL, Smith PJ, Pace RJ, Krausz E (2006b) Spectral hole burning at the low-energy absorption edge of photosystem II core complexes. J Luminesc 119–120:298–303
Ihalainen JA, Gobets B, Sznee K, Brazzoli M, Croce R, Bassi R, van Grondelle R, Korppi-Tommola JEI, Dekker JP (2000) Evidence for two spectroscopically different dimers of light- harvesting complex I from green plants. Biochemistry 39:8625–8631
Ihalainen JA, Rätsep M, Jensen PE, Scheller HV, Croce R, Bassi R, Korppi-Tommola JEI, Freiberg A (2003) Red spectral forms of chlorophylls in green plant PSI —a site-selective and high-pressure spectroscopy study. J Phys Chem B 107:9086–9093
Ilioaia C, Johnson MP, Horton P, Ruban AV (2008) Induction of efficient energy dissipation in the isolated light-harvesting complex of photosystem II in the absence of protein aggregation. J Biol Chem 283:29505–29512
Ilioaia C, Johnson MP, Liao PN, Pascal AA, van Grondelle R, Walla PJ, Ruban AV, Robert B (2011) Photoprotection in plants involves a change in lutein 1 binding domain in the major light-harvesting complex of Photosystem II. J Biol Chem 286:27247–27254
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta, Bioenerg 1817:182–193
Javorfi T, Naqvi KR, Melo TB, Bangar BR, Garab G (1996) Effect of scattering and sieving in the absorption and circular dichroism spectra of trimers and macro-aggregates of the CHL A/B light-harvesting complex, LHCII, and in thylakoid membranes. Prog Biophys & Mol Biol 65:E225
Johnson MP, Goral TK, Duffy CD, Brain AP, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23:1468–1479
Kell A, Feng X, Lin C, Reus M, Holzwarth AR, Jankowiak R (2014) On the charge-transfer character of the low-energy Chl a Qy absorption band in aggregated LHCII pigment protein complexes. J Phys Chem B 118:6086–6091
Konermann L, Gatzen G, Holzwarth AR (1997a) Primary processes and structure of the photosystem II reaction center. 5. Modeling of the fluorescence kinetics of the D1-D2-cyt-b559 complex at 77K. J Phys Chem B 101:2933–2944
Konermann L, Holzwarth AR (1996) Analysis of the absorption spectrum of photosystem II reaction centers: Temperature dependence, pigment assignment and inhomogeneous broadening. Biochemistry 35:829–842
Konermann L, Yruela I, Holzwarth AR (1997b) Pigment assignment in the absorption spectrum of the photosystem II reaction center by site selection fluorescence spectroscopy. Biochemistry 36:7498–7502
Konig C, Neugebauer J (2011) First-principles calculation of electronic spectra of light-harvesting complex II. Phys Chem Chem Phys 13:10475–10490
Krausz E, Hughes JL, Smith P, Pace R, Arsköld SP (2005) Oxygen-evolving Photosystem II core complexes: a new paradigm based on the spectral identification of the charge-separating state, the primary acceptor and assignment of low-temperature fluorescence. Photochem Photobiol Sci 4:744–753
Kröner D, Götze JP (2012) Modeling of a violaxanthin-chlorophyll b chromophore pair in its LHCII environment using CAM-B3LYP. J Photochem Photobiol, B 109:12–19
Krueger BP, Scholes GD, Fleming GR (1998) Calculation of couplings and energy-transfer pathways between the pigments of LH2 by the ab-inito transition density cube method. J Phys Chem B 102:5378–5386
Krüger TP, Ilioaia C, Johnson MP, Ruban AV, van Grondelle R (2014) Disentangling the low-energy states of the major light-harvesting complex of plants and their role in photoprotection. Biochim Biophys Acta-Bioenergetics 1837:1027–1038
Krüger TPJ, Ilioaia C, Valkunas L, van Grondelle R (2011a) Fluorescence intermittency from the main plant light-harvesting complex: sensitivity to the local environment. J Phys Chem B 115:5083–5095
Krüger TPJ, Novoderezhkin V, Ilioaia C, van Grondelle R (2010) Fluorescence spectral dynamics of single LHCII trimers. Biophys J 98:3093–3101
Krüger TPJ, Wientjes E, Croce R, van Grondelle R (2011b) Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc Natl Acad Sci USA 108:13516–13521
Kühlbrandt W (1984) Three-dimensional structure of the light-harvesting chlorophyll a/b-protein complex. Nature 307:478–480
Lambrev P, Várkonyi Z, Krumova S, Kovács L, Miloslavina Y, Holzwarth AR, Garab G (2007) Importance of trimer-trimer interactions for the native state of the plant light-harvesting complex II. Biochim Biophys Acta 1767:847–853
Lambrev PH, Nilkens M, Miloslavina Y, Jahns P, Holzwarth AR (2010) Kinetic and spectral resolution of multiple nonphotochemical quenching components in Arabidopsis leaves. Plant Physiol 152:1611–1624
Lathrop EJP, Friesner RA (1994) Simulation of optical spectra from the reaction center of Rb. sphaeroides. Effects of an internal charge-separated state of the special pair. J Phys Chem 98:3056–3066
Leuenberger M, Morris JM, Chan AM, Leonelli L, Niyogi KK, Fleming GR (2017) Dissecting and modeling zeaxanthin- and lutein-dependent nonphotochemical quenching in Arabidopsis thaliana. Proc Natl Acad Sci USA 114:E7009–E7017
Li J (1985) Light-harvesting chlorophyll a/b protein: three- dimensional structure of a reconstituted membrane lattice in negative stain. Proc Natl Acad Sci USA 82:386–390
Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Ann Rev Plant Biol 60:239–260
Liao PN, Holleboom CP, Wilk L, Kühlbrandt W, Walla PJ (2010) Correlation of Car S1 -%3eChl with Chl -%3eCar S1 energy transfer supports the excitonic model in quenched light harvesting complex II. J Physi Chem B 114:15650–15655
Liguori N, Periole X, Marrink SJ, Croce R (2015) From light-harvesting to photoprotection: structural basis of the dynamic switch of the major antenna complex of plants (LHCII). Sci Rep 5:15661
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 angstrom resolution. Nature 428:287–292
Magdaong NM, Enriquez MM, LaFountain AM, Rafka L, Frank HA (2013) Effect of protein aggregation on the spectroscopic properties and excited state kinetics of the LHCII pigment-protein complex from green plants. Photosynth Res 118:259–276
Mascoli V, Liguori N, Xu P, Roy LM, van Stokkum IHM, Croce R (2019) Capturing the quenching mechanism of light-harvesting complexes of plants by zooming in on the ensemble. Chem 5:2900–2912
Matsubara S, Förster B, Waterman M, Robinson SA, Pogson BJ, Gunning B, Osmond B (2012) From ecophysiology to phenomics: some implications of photoprotection and shade-sun acclimation in situ for dynamics of thylakoids in vitro. Philos Trans R Soc Lond B Biol Sci 367:3503–3514
Miloslavina Y, DeBianchi S, Dall'Osto L, Bassi R, Holzwarth AR (2011) Quenching in Arabidopsis thaliana mutants lacking monomeric antenna proteins of photosystem II. J Biol Chem 286:36830–36840
Miloslavina Y, Wehner A, Wientjes E, Reus M, Lambrev P, Garab G, Croce R, Holzwarth AR (2008) Far-red fluorescence: a direct spectroscopic marker for LHCII oligomers forming in non-photochemical quenching. FEBS Lett 582:3625–3631
Moore LJ, Zhou H, Boxer SG (1999) Excited-state electronic asymmetry of the special pair in photosynthetic reaction center mutants: Absorption and stark spectroscopy. Biochemistry 38:11949–11960
Morosinotto T, Breton J, Bassi R, Croce R (2003) The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J Biol Chem 278:49223–49229
Morosinotto T, Mozzo M, Bassi R, Croce R (2005) Pigment-pigment interactions in Lhca4 antenna complex of higher plants photosystem I. J Biol Chem 280:20612–20619
Mozzo M, Passarini F, Bassi R, van Amerongen H, Croce R (2008) Photoprotection in higher plants: the putative quenching site is conserved in all outer light-harvesting complexes of photosystem II. Biochim Biophys Acta 1777:1263–1267
Müh F, Madjet M, Renger T (2010) Structure-based identification of energy sinks in plant light-harvesting complex II. J Phys Chem B 114:13517–13535
Müh F, Renger T (2012) Refined structure-based simulation of plant light-harvesting complex II: linear optical spectra of trimers and aggregates. Biochim Biophys Acta 1817:1446–1460
Müller MG, Dorra D, Holzwarth AR, Gad'on N, Drews G (1995) Time-dependent radical pair relaxation in chromatophores of an antenna-free mutant from Rhodobacter capsulatus. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, vol 1. Kluwer Academic Publishers, Dordrecht, pp 595–598
Müller MG, Drews G, Holzwarth AR (1996) Primary charge separation processes in reaction centers of an antenna-free mutant of Rhodobacter capsulatus. Chem Phys Lett 258:194–202
Müller MG, Griebenow K, Holzwarth AR (1991) Primary processes in isolated photosynthetic bacterial reaction centers from Chloroflexus aurantiacus studied by picosecond fluorescence spectroscopy. Biochim Biophys Acta 1098:1–12
Müller MG, Griebenow K, Holzwarth AR (1992) Primary processes in isolated bacterial reaction centers from Rhodobacter sphaeroides studied by picosecond fluorescence kinetics. Chem Phys Lett 199:465–469
Müller MG, Lambrev P, Reus M, Wientjes E, Croce R, Holzwarth AR (2010) Singlet energy dissipation in photosystem II light-harvesting complex does not involve energy transfer to carotenoids. ChemPhysChem 11:1289–1296
Mullineaux CW, Pascal AA, Horton P, Holzwarth AR (1993) Excitation energy quenching in aggregates of the LHC II chlorophyll-protein complex: a time-resolved fluorescence study. Biochim Biophys Acta 1141:23–28
Murata N, Satoh K (1986) Absorption and fluorescence emission by intact cells, chloroplasts, and chlorophyll-protein complexes. In: Amesz J, Fork DC (eds) Govindjee. Light Emission by Plants and Bacteria. Academic Press, New York, pp 137–160
Nagy PI, Erhardt PW (2010) Theoretical studies of salt-bridge formation by amino acid side chains in low and medium polarity environments. J Phys Chem B 114:16436–16442
Naqvi KR (1998) Carotenoid-induced electronic relaxation of the first excited state of antenna chlorophylls. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol 1. Kluver Academic Publishers, Netherlands, pp 265–270
Naqvi KR, Javorfi T, Melo TB, Garab G (1999) More on the catalysis of internal conversion in chlorophyll a by an adjacent carotenoid in light-harvesting complex (Chla/b LHCII) of higher plants: time-resolved triplet-minus-singlet spectra of detergent-perturbed complexes. Spectrochim Acta A 55:193–204
Naqvi KR, Melo TB, Bangar Raju B, Javorfi T, Garab G (1997) Comparison of the absorption spectra of trimers and aggregates of chlorophyll a/b light-harvesting complex LHC II. Spectrochim Acta A 53:1925–1936
Nicol L, Nawrocki WJ, Croce R (2019) Disentangling the sites of non-photochemical quenching in vascular plants. Nat Plants 5:1177–1183
Novoderezhkin V, Marin A, van Grondelle R (2011) Intra- and inter-monomeric transfers in the light harvesting LHCII complex: the Redfield-Forster picture. Phys Chem Chem Phys 13:17093–17103
Novoderezhkin VI, Croce R, van GR, (2018) Dynamics of the mixed exciton and charge-transfer states in light-harvesting complex Lhca4: Hierarchical equation approach. Biochim Biophys Acta Bioenerg 1859:655–665
Novoderezhkin VI, Croce R, Wahadoszamen M, Polukhina I, Romero E, van Grondelle R (2016) Mixing of exciton and charge-transfer states in light-harvesting complex Lhca4. Phys Chem Chem Phys 18:19368–19377
Novoderezhkin VI, Dekker JP, van Grondelle R (2007) Mixing of exciton and charge-transfer states in Photosystem II reaction centers: Modeling of stark spectra with modified redfield theory. Biophys J 93:1293–1311
Novoderezhkin VI, Palacios MA, van Amerongen H, van Grondelle R (2005) Excitation dynamics in the LHCII complex of higher plants: Modeling based on the 2.72 angstrom crystal structure. J Phys Chem B 109:10493–10504
Ogrodnik A, Volk M, Letterer R, Feick R, Michel-Beyerle ME (1988a) Determination of free energies in reaction centers of Rb. sphaeroides. Biochim Biophys Acta 936:361–371
Ogrodnik A, Volk M, Michel-Beyerle ME (1988b) On the energetics of the states 1P,3P and P+H- in reaction centers of Rb. sphaeroides. In: Breton J, Vermeglio A (eds) The photosynthetic bacterial reaction center. Plenum Press, New York, pp 177–183
Ostroumov EE, Fadeev VV, Khristin MS, Pashchenko VZ, Tusov VB (2007) Fluorescence characteristics and photophysical parameters of light-harvesting chlorophyll a/b complex aggregates. Biophysics 52:462–467
Pace CN (2009) Energetics of protein hydrogen bonds. Nat Struct Mol Biol 16:681–682
Pace CN, Grimsley GR, Scholtz JM (2009) Protein ionizable groups: pK values and their contribution to protein stability and solubility. J Biol Chem 284:13285–13289
Pan J, Lin S, Woodbury NW (2012) Bacteriochlorophyll excited-state quenching pathways in bacterial reaction centers with the primary donor oxidized. J Phys Chem B 116:2014–2022
Pan J, Saer RG, Lin S, Guo Z, Beatty JT, Woodbury NW (2013) The protein environment of the bacteriopheophytin anion modulates charge separation and charge recombination in bacterial reaction centers. J Phys Chem B 117:7179–7189
Pascal AA, Liu ZF, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang WR, Ruban AV (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Pawlak K, Paul S, Liu C, Reus M, Yang C, Holzwarth AR (2020) On the PsbS-induced quenching in the major light-harvesting complex LHCII studied inproteoliposomes. Photosyn Res. https://doi.org/10.1007/s11120-020-00740-z
Pieper J, Irrgang K-D, Rätsep M, Schrötter T, Voigt J, Small GJ, Renk G, Jankowiak R (1999a) Effects of aggregation on trimeric light-harvesting complex II of green plants: A hole-burning study. J Phys Chem A 103:2422–2428
Pieper J, Rätsep M, Jankowiak R, Irrgang K-D, Voigt J, Renger G, Small GJ (1999b) Q(Y)-level structure and dynamics of solubilized light- harvesting complex II of green plants: Pressure and hole burning studies. J Phys Chem A 103:2412–2421
Pieper J, Schödel R, Irrgang K-D, Voigt J, Renger G (2001) Electron-phonon coupling in solubilized LHC II complexes of green plants investigated by line-narrowing and temperature-dependent fluorescence spectroscopy. J Phys Chem B 105:7115–7124
Ramanan C, Gruber JM, Maly P, Negretti M, Novoderezhkin V, Kruger TP, Mancal T, Croce R, van Grondelle R (2015) The role of exciton delocalization in the major photosynthetic light-harvesting antenna of plants. Biophys J 108:1047–1056
Rätsep M, Johnson TW, Chitnis PR, Small GJ (2000) The red-absorbing chlorophyll a antenna states of photosystem I: a hole-burning study of Synechocystis sp PCC 6803 and its mutants. J Phys Chem B 104:836–847
Robert B, Horton P, Pascal AA, Ruban AV (2004) Insights into the molecular dynamics of plant light-harvesting proteins in vivo. Trends Plant Sci 9:385–390
Roelofs TA, Gilbert M, Shuvalov VA, Holzwarth AR (1991) Picosecond fluorescence kinetics of the D1-D2-cyt-b559 photosystem II reaction center complex. Energy Transfer and primary charge separation processes. Biochim Biophys Acta 1060:237–244
Roelofs TA, Kwa SLS, van Grondelle R, Dekker JP, Holzwarth AR (1993) Primary processes and structure of the photosystem II reaction center: II. Low-temperature picosecond fluorescence kinetics of a D1-D2-cyt-b-559 reaction center complex isolated by short Triton exposure. Biochim Biophys Acta 1143:147–157
Romero E, Mozzo M, van Stokkum IHM, Dekker JP, van Grondelle R, Croce R (2009) The origin of the low-energy form of photosystem I light-harvesting complex Lhca4: mixing of the lowest exciton with a charge-transfer state. Biophys J 96:L35–L37
Ruban AV (2015) Evolution under the sun: optimizing light harvesting in photosynthesis. J Exp Bot 66:7–23
Ruban AV (2016) Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol 170:1903–1916
Ruban AV (2019) The mechanism of nonphotochemical quenching: the end of the ongoing debate. Plant Physiol 181:383–384
Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Ruban AV, Dekker JP, Horton P, van Grondelle R (1995a) Temperature dependence of chlorophyll fluorescence from the light harvesting complex II of higher plants. Photochem Photobiol 61:216–221
Ruban AV, Horton P (1992) Mechanism of DpH-dependent dissipation of absorbed excitation energy by photosynthetic membranes. 1. Spectroscopic analysis of isolated light-harvesting complexes. Biochim Biophys Acta-Bioenergetics 1102:30–38
Ruban AV, Horton P, Robert B (1995b) Resonance raman spectroscopy of the photosystem II light- harvesting complex of green plants: A comparison of trimeric and aggregated. Biochemistry 34:2333–2337
Ruban AV, Rees D, Noctor GD, Young A, Horton P (1991) Long-wavelength chlorophyll species are associated with amplification of high-energy-state excitation quenching in higher plants. Biochim Biophys Acta 1059:355–360
Schulman SG (1985) Molecular luminescence spectroscopy. In: Elving PJ, Winefordner JP (eds) Chemical Analysis, vol 77. Wiley, New York, pp 1–27
Segatta F, Cupellini L, Garavelli M, Mennucci B (2019) Quantum Chemical Modeling of the Photoinduced Activity of Multichromophoric Biosystems: Focus Review. Chem Rev 119:9361–9380
Standfuss J, van Scheltinga ACT, Lamborghini M, Kühlbrandt W (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5Å resolution. EMBO J 24:919–928
Su X, Ma J, Wei X, Cao P, Zhu D, Chang W, Liu Z, Zhang X, Li M (2017) Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 357:815–820
Szczepaniak M, Sander J, Nowaczyk M, Müller MG, Rögner M, Holzwarth AR (2008) Influence of the protein environment on the regulation of the Photosystem II activity—a time-resolved fluorescence study. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis energy from the sun. Springer, Dordrecht, pp 211–214
Szczepaniak M, Sander J, Nowaczyk M, Müller MG, Rögner M, Holzwarth AR (2009) Charge separation, stabilization, and protein relaxation in photosystem II core particles with closed reaction center. Biophys J 96:621–631
Thapper A, Mamedov F, Mokvist F, Hammarström L, Styring S (2009) Defining the far-red limit of photosystem II in spinach. Plant Cell 21:2391–2401
Tutkus M, Akhtar P, Chmeliov J, Görföl F, Trinkunas G, Lambrev PH, Valkunas L (2018) Fluorescence microscopy of single liposomes with incorporated pigment-proteins. Langmuir 34:14410–14418
van Amerongen H, Croce R (2013) Light harvesting in photosystem II. Photosynth Res 116:251–263
van Amerongen H, van Grondelle R (2001) Understanding the energy transfer function of LHCII, the major light-harvesting complex of green plants. J Phys Chem B 105:604–617
van Leeuwen PJ, Nieveen MC, van de Meent EJ, Dekker JP, van Gorkom HJ (1991) Rapid and simple isolation of pure photosystem II core and reaction center particles from spinach. Photosynth Res 28:149–153
van Oort B, Roy LM, Xu P, Lu Y, Karcher D, Bock R, Croce R (2018) Revisiting the role of xanthophylls in nonphotochemical quenching. J Phys Chem Lett 9:346–352
Volk M, Häberle T, Feick R, Ogrodnik A, Michel-Beyerle ME (1993) What can be learned from the singlet-triplet splitting of the radical pair P+H- in the photosynthetic reaction center—conclusions from electric field effects on the P+H- recombination dynamics. J Phys Chem 97:9831–9836
Wahadoszamen M, Berera R, Ara AM, Romero E, van Grondelle R (2012) Identification of two emitting sites in the dissipative state of the major light harvesting antenna. Phys Chem Chem Phys 14:759–766
Wang HY, Lin S, Allen JP, Williams JC, Blankert S, Laser C, Woodbury NW (2007) Protein dynamics control the kinetics of initial electron transfer in photosynthesis. Science 316:747–750
Wang J, Cieplak P, Kollman PA (2000) How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem 21:1049–1074
Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z (2016) Structure of spinach photosystem II-LHCII supercomplex at 3.2 A resolution. Nature 534:69–74
Wormit M, Harbach PHP, Mewes JM, Amarie S, Wachtveitl J, Dreuw A (2009) Excitation energy transfer and carotenoid radical cation formation in light harvesting complexes; a theoretical perspective. Biochim Biophys Acta-Bioenergetics 1787:738–746
Yakushevska AE, Keegstra W, Boekema EJ, Dekker JP, Andersson J, Jansson S, Ruban AV, Horton P (2003) The structure of photosystem II in Arabidopsis: Localization of the CP26 and CP29 antenna complexes. Biochemistry 42:608–613
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Yang Y, Jankowiak R, Lin C, Pawlak K, Reus M, Holzwarth AR, Li J (2014) Effect of the LHCII pigment-protein complex aggregation on photovoltaic properties of sensitized TiO2 solar cells. PCCP 16:20856–20865
Zhou H, Boxer SG (1997) Charge resonance effects on electronic absorption line shapes: application to the heterodimer absorption of bacterial photosynthetic reaction centers. J Phys Chem B 101:5759–5766
Zhou H, Boxer SG (1998) Probing excited-state electron transfer by resonance Stark spectroscopy. 2. Theory and application. J Phys Chem B 102:9148–9160
Acknowledgements
This research was supported by grants to ARH from the Deutsche Forschungsgemeinschaft (DFG HO-924/3–1 and in part by SFB 663), and by the EU Training and Research Network “Harvest” of the European Union. PHL acknowledges support from the Hungarian National Research, Development and Innovation Fund (grants NN 124904, 2018–1.2.1-NKP-2018–00009). JPG is currently funded by the DFG, project no. 393271229. We thank Prof. W. Kühlbrandt and Dr. Tiago Barros (Max-Planck-Institute for Biophysics, Frankfurt a. Main, Germany) for providing the LHCII crystals. We also thank the Max-Planck-Institute for Chemical Energy Conversion for generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ostroumov, E.E., Götze, J.P., Reus, M. et al. Characterization of fluorescent chlorophyll charge-transfer states as intermediates in the excited state quenching of light-harvesting complex II. Photosynth Res 144, 171–193 (2020). https://doi.org/10.1007/s11120-020-00745-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00745-8