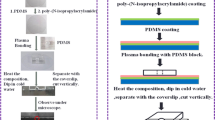
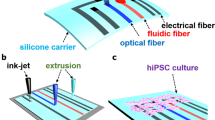
Abstract
Muscular thin films (MTFs), have already found a variety of applications in cardiac tissue engineering and in building of lab-on-a-chip systems. Here we present a novel approach to label-free mapping of excitation waves in the cardiomyocyte cell cultures with the use of MTFs. Neonatal rat ventricular cardiomyocytes were cultured on polydimethylsiloxane (PDMS) thin films and observed by means of off-axis illumination. Inflexions of the membrane created by the contraction of cardiomyocytes led to formation of patterns of bright and dark areas on the surface of the membrane. These patterns were recorded and analyzed for the monitoring of the contraction propagation. The method was compared with a standard optical mapping technique based on the use of a Ca2+-sensitive fluorescent dye. A good consistency of the results obtained by these two methods was demonstrated. The proposed method is non-toxic and might be of particular interest for the purpose of continuous monitoring in test systems based on human induced pluripotent stem cells.








Similar content being viewed by others
Abbreviations
- HiPSC:
-
Human induced pluripotent stem cells
- iPSC:
-
Induced pluripotent stem cells
- MEAs:
-
Multi-electrode arrays
- MEMs:
-
Microelectromechanical systems
- MTFs:
-
Muscular thin films
- PDMS:
-
Polydimethylsiloxane
- SEM:
-
Scanning electron microscopy
- AFM:
-
Atomic-force microscopy
References
Agarwal, A., J. A. Goss, A. Cho, M. L. McCain, and K. K. Parker. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab. Chip 13:3599–3608, 2013.
Ahola, A., A. L. Kiviaho, K. Larsson, M. Honkanen, K. Aalto-Setälä, and J. Hyttinen. Video image-based analysis of single human induced pluripotent stem cell derived cardiomyocyte beating dynamics using digital image correlation. Biomed. Eng. Online 13:39, 2014.
Ahola, A., R. P. Pölönen, K. Aalto-Setälä, and J. Hyttinen. Simultaneous measurement of contraction and calcium transients in stem cell derived cardiomyocytes. Ann. Biomed. Eng. 46:148–158, 2018.
Alford, P. W., A. W. Feinberg, S. P. Sheehy, and K. K. Parker. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials 31:3613–3621, 2010.
Benam, K. H., S. Dauth, B. Hassell, A. Herland, A. Jain, K.-J. Jang, K. Karalis, H. J. Kim, L. MacQueen, R. Mahmoodian, S. Musah, Y. S. Torisawa, A. D. van der Meer, R. Villenave, M. Yadid, K. K. Parker, and D. E. Ingber. Engineered in vitro disease models. Annu. Rev. Pathol. Mech. Dis. 10:195–262, 2015.
Bingen, B. O., M. C. Engels, M. J. Schalij, W. Jangsangthong, Z. Neshati, I. Feola, D. L. Ypey, S. F. Askar, A. V. Panfilov, D. A. Pijnappels, and A. A. de Vries. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc. Res. 104:194–205, 2014.
Boudreau-Beland, J., J. E. Duverger, E. Petitjean, A. Maguy, J. Ledoux, and P. Comtois. Spatiotemporal stability of neonatal rat cardiomyocyte monolayers spontaneous activity is dependent on the culture substrate. PLoS ONE 10(6):e0127977, 2015.
Braunwald, E. Heart Disease, a Textbook of Cardiovascular Medicine. Philadelphia, PA: WB Saunders Company, 2001.
Burton, R. A. B., A. Klimas, C. M. Ambrosi, J. Tomek, A. Corbett, E. Entcheva, and G. Bub. Optical control of excitation waves in cardiac tissue. Nat. Photonics 9:813–816, 2015.
Capulli, A. K., K. Tian, N. Mehandru, A. Bukhta, S. F. Choudhury, M. Suchyta, and K. K. Parker. Approaching the in vitro clinical trial: engineering organs on chips. Lab Chip 14:3181–3186, 2014.
Chae, S. K., J. H. Ryoo, and S. H. Lee. Thin and large free-standing PDMS membrane by using polystyrene Petri dish. Biochip J. 6:184–190, 2012.
Cherry, E. M., and F. H. Fenton. Visualization of spiral and scroll waves in simulated and experimental cardiac tissue. New J. Phys. 10:125016, 2008.
Christoph, J., and S. Luther. Marker-free tracking for motion artifact compensation and deformation measurements in optical mapping videos of contracting hearts. Front. Physiol. 9:1483, 2018.
Christoph, J., J. Schröder-Schetelig, and S. Luther. Electromechanical optical mapping. Prog. Biophys. Mol. Biol. 130:150–169, 2017.
Davidenko, J. M., A. V. Pertsov, R. Salomonsz, W. Baxter, and J. Jalife. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 355:349–351, 1992.
del Álamo, J. C., D. Lemons, R. Serrano, A. Savchenko, F. Cerignoli, R. Bodmer, and M. Mercola. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochem. Biophys. Acta 1717–1727:2016, 1863.
Denning, C., V. Borgdorff, J. Crutchley, K. S. A. Firth, V. George, S. Kalra, A. Kondrashov, M. D. Hoang, D. Mosqueira, A. Patel, and L. Prodanov. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 1728–1748:2016, 1863.
Dimitriadis, E. K., F. Horkay, J. Maresca, B. Kachar, and R. S. Chadwick. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 82(5):2798–2810, 2002.
Feinberg, A. W., A. Feigel, S. S. Shevkoplyas, S. Sheehy, G. M. Whitesides, and K. K. Parker. Muscular thin films for building actuators and powering devices. Science 317:1366–1370, 2007.
Goßmann, M., R. Frotscher, P. Linder, S. Neumann, R. Bayer, M. Epple, M. Staat, A. Artmann, and G. M. Artmann. Mechano-pharmacological characterization of cardiomyocytes derived from human induced pluripotent stem cells. Cell. Physiol. Biochem. 38:1182–1198, 2016.
Grosberg, A., P. W. Alford, M. L. McCain, and K. K. Parker. Ensembles of engineered cardiac tissues for physiological and pharmacological study. Heart on a chip. Lab. Chip. 11:4165–4173, 2011.
Hardy, M. E. L., C. L. Lawrence, N. B. Standen, and G. C. Rodrigo. Can optical recordings of membrane potential be used to screen for drug-induced action potential prolongation in single cardiac myocytes? J. Pharmacol. Toxicol. Methods 54:173–182, 2006.
Hayakawa, T., T. Kunihiro, S. Dowaki, H. Uno, E. Matsui, M. Uchida, S. Kobayashi, A. Yasuda, T. Shimizu, and T. Okano. Noninvasive evaluation of contractile behavior of cardiomyocyte nonolayers based on motion vector analysis. Tissue Eng. Part C 18:21–32, 2012.
Hecht, E. Optics. Harlow: Pearson Education, p. 109, 2015.
Herron, T. J., P. Lee, and J. Jalife. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 110:609–623, 2012.
Herron, T. J., A. M. D. Rocha, K. F. Campbell, D. Ponce-Balbuena, B. C. Willis, G. Guerrero-Serna, Q. Liu, M. Klos, H. Musa, M. Zarzoso, and A. Bizy. Extracellular matrix–mediated maturation of human pluripotent stem cell–derived cardiac monolayer structure and electrophysiological function. Circulation 9(4):e003638, 2016.
Hirt, M. N., A. Hansen, and T. Eschenhagen. Cardiac tissue engineering: state of the art. Circ. Res. 114:354–367, 2014.
Hossain, M. M., E. Shimizu, M. Saito, S. Ramachandra Rao, Y. Yamaguchi, and E. Tamiya. Non-invasive characterization of mouse embryonic stem cell derived cardiomyocytes based on the intensity variation in digital beating video. Analyst 135:1624–1630, 2010.
Huebsch, N., P. Loskill, M. A. Mandegar, N. C. Marks, A. S. Sheehan, Z. Ma, A. Mathur, T. N. Nguyen, J. C. Yoo, L. M. Judge, and C. I. Spencer. Automated video-based analysis of contractility and calcium flux in human-induced pluripotent stem cell-derived cardiomyocytes cultured over different spatial scales. Tissue Eng. Part C 21:467–479, 2015.
Hwang, S.-M., K.-H. Yea, and K. J. Lee. Regular & alternant. Spiral waves of contractile motion on rat ventricle cell cultures. Phys. Rev. Lett. 92:1–4, 2004.
Jacot, J. G., A. D. McCulloch, and J. H. Omens. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 95(7):3479–3487, 2008.
Jalife, J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu. Rev. Physiol. 62:25–50, 2000.
Kadota, S., I. Minami, N. Morone, J. E. Heuser, K. Agladze, and N. Nakatsuji. Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur. Heart J. 34:1147–1156, 2013.
Kamgoué, A., J. Ohayon, Y. Usson, L. Riou, and P. Tracqui. Quantification of cardiomyocyte contraction based on image correlation analysis. Cytom. Part A 75:298–308, 2009.
Kijlstra, J. D., D. Hu, N. Mittal, E. Kausel, P. Van Der Meer, A. Garakani, and I. J. Domian. Integrated analysis of contractile kinetics, force generation, and electrical activity in single human stem cell-derived cardiomyocytes. Stem Cell Rep. 5:1226–1238, 2015.
Kim, S. B., H. Bae, J. M. Cha, S. J. Moon, M. R. Dokmeci, D. M. Cropek, and A. Khademhosseini. A cell-based biosensor for real-time detection of cardiotoxicity using lensfree imaging. Lab. Chip. 11:1801–1807, 2011.
Kléber, A. G., and Y. Rudy. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 84:431–488, 2004.
Kudryashova, N. N., A. S. Teplenin, Y. V. Orlova, L. V. Selina, and K. Agladze. Arrhythmogenic role of the border between two areas of cardiac cell alignment. J. Mol. Cell. Cardiol. 76:227–234, 2014.
Laurila, E., A. Ahola, J. Hyttinen, and K. Aalto-Setälä. Methods for in vitro functional analysis of iPSC derived cardiomyocytes—special focus on analyzing the mechanical beating behavior. Biochem. Biophys. Acta 1864–1872:2016, 1863.
Linder, P., J. Trzewik, M. Rüffer, G. M. Artmann, I. Digel, R. Kurz, A. Rothermel, A. Robitzki, and A. Temiz Artmann. Contractile tension and beating rates of self-exciting monolayers and 3D-tissue constructs of neonatal rat cardiomyocytes. Med. Biol. Eng. Comput. 48:59–65, 2010.
McDonald, J. C., and G. M. Whitesides. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 35:491–499, 2002.
Moon, I., E. Ahmadzadeh, K. Jaferzadeh, and N. Kim. Automated quantification study of human cardiomyocyte synchronization using holographic imaging. Biomed. Opt. Express 10:610–621, 2019.
Nawroth, J. C., H. Lee, A. W. Feinberg, C. M. Ripplinger, M. L. McCain, A. Grosberg, J. O. Dabiri, and K. K. Parker. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 30:792–797, 2012.
Nyapshaev, I. A., A. V. Ankudinov, A. V. Stovpyaga, E. Y. Trofimova, and M. Y. Eropkin. Diagnostics of living cells under an atomic force microscope using a submicron spherical probe with a calibrated radius of curvature. Tech. Phys. 57(10):1430–1437, 2012.
Orlova, Y., N. Magome, L. Liu, Y. Chen, and K. Agladze. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 32:5615–5624, 2011.
Park, S. J., M. Gazzola, K. S. Park, S. Park, V. Di Santo, E. L. Blevins, J. U. Lind, P. H. Campbell, S. Dauth, A. K. Capulli, and F. S. Pasqualini. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 353:158–162, 2016.
Pauwelyn, T., V. Reumers, G. Vanmeerbeeck, R. Stahl, S. Janssens, L. Lagae, D. Braeken, and A. Lambrechts. Label-free cardiac contractility monitoring for drug screening applications based on compact high-speed lens-free imaging. Proc. SPIE 9328:932818, 2015.
Pauwelyn, T., R. Stahl, L. Mayo, X. Zheng, A. Lambrechts, S. Janssens, L. Lagae, V. Reumers, and D. Braeken. Reflective lens-free imaging on high-density silicon microelectrode arrays for monitoring and evaluation of in vitro cardiac contractility. Biomed. Opt. Express 9:1827–1841, 2018.
Podgurskaya, A. D., V. A. Tsvelaya, S. R. Frolova, I. Y. Kalita, N. N. Kudryashova, and K. I. Agladze. Effect of heptanol and ethanol on excitation wave propagation in a neonatal rat ventricular myocyte monolayer. Toxicol. Vitrol. 51:136–144, 2018.
Podgurskaya, A. D., V. A. Tsvelaya, M. M. Slotvitsky, E. V. Dementyeva, K. R. Valetdinova, and K. I. Agladze. The Use of iPSC-derived cardiomyocytes and optical mapping for erythromycin arrhythmogenicity testing. Cardiovasc. Toxicol. 19:518–528, 2019.
Sackmann, E. K., A. L. Fulton, and D. J. Beebe. The present and future role of microfluidics in biomedical research. Nature 507:181–189, 2014.
Schaffer, P., H. Ahammer, W. Müller, B. Koidl, and H. Windisch. Di-4-ANEPPS causes photodynamic damage to isolated cardiomyocytes. Pflügers Arch. Eur. J. Physiol. 426:548–551, 1994.
Shaked, N. T., L. L. Satterwhite, N. Bursac, and A. Wax. Whole-cell-analysis of live cardiomyocytes using wide-field interferometric phase microscopy. Biomed. Opt. Express 1:1165–1167, 2010.
Shutko, A. V., V. S. Gorbunov, K. G. Guria, and K. I. Agladze. Biocontractile microfluidic channels for peristaltic pumping. Biomed. Microdev. 19:72, 2017.
Teplenin, A., A. Krasheninnikova, N. Agladze, K. Sidoruk, O. Agapova, I. Agapov, V. Bogush, and K. Agladze. Functional analysis of the engineered cardiac tissue grown on recombinant spidroin fiber meshes. PLoS ONE 10:e0121155, 2015.
Wang, Y., P. Kanjanaboos, S. P. McBride, E. Barry, X. M. Lin, and H. M. Jaeger. Mechanical properties of self-assembled nanoparticle membranes: stretching and bending. Faraday Discuss. 181:325–338, 2015.
Wang, L., L. Liu, X. Li, N. Magome, K. Agladze, and Y. Chen. Multi-electrode monitoring of guided excitation in patterned cardiomyocytes. Microelectron. Eng. 111:267–271, 2013.
Whitesides, G. M. The origins and the future of microfluidics. Nature 442:368–373, 2006.
Winfree, A. T. When Time Breaks Down: The Three-Dimensional Dynamics of Electrochemical Waves and Cardiac Arrhythmias. New Jersey: Princeton University Press, 1987.
Wolfe, D. B., D. Qin, and G. M. Whitesides. Rapid prototyping of microstructures by soft lithography for biotechnology. Methods Mol. Biol. 583:81–107, 2010.
Acknowledgments
This research work was partially supported by the Russian Foundation for Basic Research grant 16-34-60225 and by the Ministry of Education and Science of the Russian Federation Grant (state task) 6.9906.2017/BCh. Authors are grateful to the Center for collective use of unique scientific equipment in the field of nanotechnology of Moscow Institute of Physics and Technology and personally to Dr. Negrov D.V. for the preparation of micro-patterned molds used in this research.
Author Contributions
VAB, VSG, KGG and KIA designed the experiments. VAB &VSG performed the experiments under the direction of KGG; VAB, VSG, KGG and KIA analyzed the data and contributed to discussion. VAB, VSG, KGG and KIA wrote and edited the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Smadar Cohen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1 Video 1: Label-free monitoring of excitation wave propagation in a cell culture seeded on a thin film: A – raw data; B – contrast enhanced video. (AVI 15295 kb)
S2 Video 2: Label-free monitoring of spiral wave in an isotropic cardiac culture interacting with a circular wave: A – raw data; B – contrast enhanced video. (AVI 6502 kb)
S3 Video 3: Successive mapping of the same sample by two different methods: A – Ca2+-sensitive fluorescent mapping; B – label-free mapping. (AVI 21,065 kb)
S4 Video 4: Label-free monitoring of excitation waves performed on GoPro camera: A – raw data; B – contrast enhanced video. (AVI 6776 kb)
S5 Video 5: A spiral wave in anisotropic cardiac culture observed with the label-free method: A – raw data; B – contrast enhanced video. (AVI 10,710 kb)
10439_2020_2513_MOESM6_ESM.tif
Supplementary Figure 1. Activation maps of spiral waves imaged by label-free optical mapping. (a) Activation map of the spiral wave in isotropic cardiomyocyte culture interacting with the plane wave. (b) - Activation map of the anisotropic contraction spiral wave in the patterned cardiac monolayer. Scale bars – 3 mm. (TIFF 1207 kb)
10439_2020_2513_MOESM7_ESM.tif
Supplementary Figure 2. Conduction velocities for the samples cultured on membranes with different thicknesses. (TIFF 93 kb)
10439_2020_2513_MOESM8_ESM.tif
Supplementary Figure 3. Isochrones of excitation wave propagation in cardiac culture imaged by label-free method. (a) – Wave propagation without lidocaine. (b) – Wave propagation after treatment with 2 mM lidocaine. Time interval between isochrones is equal to 30 ms. Scale bar – 2 mm. (TIFF 259 kb)
Rights and permissions
About this article
Cite this article
Balashov, V.A., Gorbunov, V.S., Guria, K.G. et al. Muscular Thin Films for Label-Free Mapping of Excitation Propagation in Cardiac Tissue. Ann Biomed Eng 48, 2425–2437 (2020). https://doi.org/10.1007/s10439-020-02513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02513-0