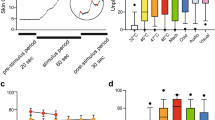
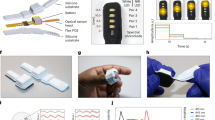
Abstract
neuECG, the simultaneous noninvasive recording of ECG and skin sympathetic nerve activity (SKNA), directly records sympathetic nerve activity over a long period of time. It can be used to measure sympathetic tone in healthy subjects and in subjects with non-cardiovascular diseases. The electrical activity that can be measured on the surface of the skin originates from the heart, the muscle or nerve structures. Because the frequency content of nerve activity falls in a higher frequency range than that of the ECG and myopotential, it is possible to use high-pass or band-pass filtering to specifically isolate the SKNA. neuECG is voltage calibrated and does not require invasive procedures to impale electrodes in nerves and thus has advantages over microneurography. Here, we present a protocol that takes <10 min to set up. The neuECG can be continuously recorded over a 24-h period or longer. We also describe methods to efficiently analyze neuECG from humans using commercially available hardware and software to facilitate adoption of this technology in clinical research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
All data described in the article will be made available upon reasonable request to the corresponding author.
References
Chen, P. S., Chen, L. S., Fishbein, M. C., Lin, S. F. & Nattel, S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114, 1500–1515 (2014).
Hart, E. C. et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am. J. Physiol. Heart Circ. Physiol. 312, H1031––H1051 (2017).
Doytchinova, A. et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 14, 25–33 (2017).
Uradu, A. et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 14, 964–971 (2017).
Kusayama, T. et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight 4, e125853 (2019).
Kabir, R. A. et al. Crescendo skin sympathetic nerve activity and ventricular arrhythmia. J. Am. Coll. Cardiol. 70, 3201–3202 (2017).
Zhang, P. et al. Characterization of skin sympathetic nerve activity in patients with cardiomyopathy and ventricular arrhythmia. Heart Rhythm 16, 1669–1675 (2019).
Kligfield, P. & Okin, P. M. Prevalence and clinical implications of improper filter settings in routine electrocardiography. Am. J. Cardiol. 99, 711–713 (2007).
McAuley, J. H., Rothwell, J. C. & Marsden, C. D. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp. Brain Res. 114, 525–541 (1997).
Komi, P. V. & Tesch, P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 42, 41–50 (1979).
Mark, A. L., Victor, R. G., Nerhed, C. & Wallin, B. G. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ. Res. 57, 461–469 (1985).
Vallbo, A. B., Hagbarth, K. E. & Wallin, B. G. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J. Appl. Physiol. (1985) 96, 1262–1269 (2004).
Robinson, E. A. et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J. Cardiovasc. Electrophysiol. 26, 70–78 (2015).
Jiang, Z. et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm 12, 1324–1332 (2015).
Doytchinova, A. et al. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm 12, 612–620 (2015).
Victor, R. G., Seals, D. R. & Mark, A. L. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J. Clin. Invest. 79, 508–516 (1987).
Tan, A. Y. et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 118, 916–925 (2008).
Ogawa, M. et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J. Am. Coll. Cardiol. 50, 335–343 (2007).
Ogawa, M. et al. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm 6, 1772–1779 (2009).
Chinda, K. et al. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm 13, 771–780 (2016).
Yuan, Y. et al. Left cervical vagal nerve stimulation reduces skin sympathetic nerve activity in patients with drug resistant epilepsy. Heart Rhythm 14, 1771–1778 (2017).
Hagbarth, K. E., Hallin, R. G., Hongell, A., Torebjork, H. E. & Wallin, B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol. Scand. 84, 164–176 (1972).
Liu, X. et al. Effects of anesthetic and sedative agents on sympathetic nerve activity. Heart Rhythm 16, 1875–1882 (2019).
Yang, L., Tang, J., Dong, J. & Zheng, J. Alpha2-adrenoceptor-independent inhibition of acetylcholine receptor channel and sodium channel by dexmedetomidine in rat superior cervical ganglion neurons. Neuroscience 289, 9–18 (2015).
Gertler, R., Brown, H. C., Mitchell, D. H. & Silvius, E. N. Dexmedetomidine: a novel sedative-analgesic agent. Proc. (Bayl. Univ. Med. Cent.) 14, 13–21 (2001).
Ebert, T. J., Muzi, M., Berens, R., Goff, D. & Kampine, J. P. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology 76, 725–733 (1992).
Sellgren, J., Ponten, J. & Wallin, B. G. Characteristics of muscle nerve sympathetic activity during general anaesthesia in humans. Acta Anaesthesiol. Scand. 36, 336–345 (1992).
Kusayama, T. et al. Skin sympathetic nerve activity and ventricular rate control during atrial fibrillation. Heart Rhythm https://doi.org/10.1016/j.hrthm.2019.11.017 (2019).
Madias, J. E. A proposal for a noninvasive monitoring of sympathetic nerve activity in patients with takotsubo syndrome. Med. Hypotheses 109, 97–101 (2017).
Ikeda, T. et al. Augmented single-unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. J. Physiol. 590, 509–518 (2012).
Scherrer, U. et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N. Engl. J. Med. 323, 693–699 (1990).
Converse, R. L. Jr. et al. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 327, 1912–1918 (1992).
Hansen, J. & Victor, R. G. Direct measurement of sympathetic activity: new insights into disordered blood pressure regulation in chronic renal failure. Curr. Opin. Nephrol. Hypertens. 3, 636–643 (1994).
Jacobsen, T. N. et al. Effects of intranasal cocaine on sympathetic nerve discharge in humans. J. Clin. Invest. 99, 628–634 (1997).
Fadel, P. J., Zhao, W. & Thomas, G. D. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J. Physiol. 549, 243–253 (2003).
Goya, T. T. et al. Increased muscle sympathetic nerve activity and impaired executive performance capacity in obstructive sleep apnea. Sleep 39, 25–33 (2016).
Nagata, Y. Flecainide augments muscle sympathetic nerve activity in humans. Circ. J. 66, 377–381 (2002).
White, D. W., Shoemaker, J. K. & Raven, P. B. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton. Neurosci. 193, 12–21 (2015).
Sanders, P., Kistler, P. M., Morton, J. B., Spence, S. J. & Kalman, J. M. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation 110, 897–903 (2004).
Hocini, M. et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 108, 1172–1175 (2003).
Piccirillo, G. et al. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm 6, 546–552 (2009).
Chan, Y. H. et al. Subcutaneous nerve activity is more accurate than heart rate variability in estimating cardiac sympathetic tone in ambulatory dogs with myocardial infarction. Heart Rhythm 12, 1619–1627 (2015).
Greaney, J. L., Stanhewicz, A. E., Kenney, W. L. & Alexander, L. M. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J. Physiol. 593, 2199–2211 (2015).
Zegarra-Parodi, R. et al. Assessment of skin blood flow following spinal manual therapy: a systematic review. Man. Ther. 20, 228–249 (2015).
Muller, M. D., Sauder, C. L. & Ray, C. A. Mental stress elicits sustained and reproducible increases in skin sympathetic nerve activity. Physiol. Rep. 1, e00002 (2013).
Bach, D. R. A head-to-head comparison of SCRalyze and Ledalab, two model-based methods for skin conductance analysis. Biol. Psychol. 103, 63–68 (2014).
Brown, R. & Macefield, V. G. Skin sympathetic nerve activity in humans during exposure to emotionally-charged images: sex differences. Front. Physiol. 5, 111 (2014).
Beurrier, C., Congar, P., Bioulac, B. & Hammond, C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J. Neurosci. 19, 599–609 (1999).
Gerstein, G. L. & Mandelbrot, B. Random walk models for the spike activity of a single neuron. Biophys. J. 4, 41–68 (1964).
Acknowledgements
This study was supported in part by NIH Grants R01HL134864 (Y.-M.C.), R01AG049924 (D.R.W.), R42DA043391 (T.H.E.) and R56HL71140, TR002208-01, R01HL139829 and 1OT2OD028190 (P.-S.C.); a Charles Fisch Cardiovascular Research Award endowed by Dr. Suzanne B. Knoebel of the Krannert Institute of Cardiology (T.K. and T.H.E.); a Medtronic-Zipes Endowment and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative (P.-S.C.); and a Cardiovascular Prospective Award from Mayo Clinic (Y.-M.C.). We thank Matthew D. Podczerwinski of Rose Hulman Ventures for signals shown in Fig. 1a.
Author information
Authors and Affiliations
Contributions
T.K., A.D., K.D. and D.R.W. performed the experiments. T.K., J.W., X.L., W.H. E.A.R., D.E.A., K.D., C.C., M.-Y.D., Y.T., P.Z., D.E., R.H.R., and M.C. analyzed the data. T.K., L.S.C., S.-F.L., R.G.V., Y.-M.C., D.R.W., T.H.E., and P.-S.C. designed the study. All authors participated in manuscript preparation and have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
Indiana University was awarded US patent no. 10,448,852 for inventing neuECG recording. The authors are willing to help and advise basic and clinical investigators who are interested in using these recording methods to study sympathetic tone.
Additional information
Peer review information Nature Protocols thanks John E. Madias, Stavros Stavrakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Doytchinova, A. et al. Heart Rhythm 14, 25–33 (2017): https://doi.org/10.1016/j.hrthm.2016.09.019
Kusayama, T. et al. JCI Insight 4, e125853 (2019): https://doi.org/10.1172/jci.insight.125853
Liu, X. et al. Heart Rhythm 16, 1875–1882 (2019): https://doi.org/10.1016/j.hrthm.2019.06.017
Supplementary information
Supplementary Video 1
Actual data acquisition of neuECG. This video shows the actual neuECG recording in a subject with premature ventricular contractions. After baseline recording, the subject placed his right hand in a bucket of ice water for 2 min, which was associated with increases in aSKNA and HR. After CPT, aSKNA and HR decreased.
Supplementary Data 1
LabChart channel settings for single-channel recording using Lead 1. This file includes six channels for neuECG data acquisition and analyses using Lead 1. It was used to record Supplementary Video 1.
Supplementary Data 2
Original neuECG data recorded during Supplementary Video 1.
Supplementary Data 3
LabChart channel settings for Leads 1 and 2. This file includes 10 channel settings for neuECG data acquisition and analyses using Leads 1 and 2.
Supplementary Data 4
Original neuECG data from Leads 1 and 2. This file includes raw neuECG data using Leads 1 and 2 to demonstrate the LabChart data analyses.
Supplementary Data 5
LabChart data summary. This file includes the summary of LabChart analyses in Steps 25–31 using Supplementary Data 3 and 4.
Supplementary Data 6
SKNA burst analyses. This file includes the SKNA burst analyses using Excel software.
Rights and permissions
About this article
Cite this article
Kusayama, T., Wong, J., Liu, X. et al. Simultaneous noninvasive recording of electrocardiogram and skin sympathetic nerve activity (neuECG). Nat Protoc 15, 1853–1877 (2020). https://doi.org/10.1038/s41596-020-0316-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0316-6
This article is cited by
-
Safety and efficacy of cardioneuroablation for vagal bradycardia in a single arm prospective study
Scientific Reports (2024)
-
Transcutaneous electrical vagus nerve stimulation to suppress premature ventricular complexes (TREAT PVC): study protocol for a multi-center, double-blind, randomized controlled trial
Trials (2023)
-
Skin sympathetic nerve activity as a potential biomarker for overactive bladder
World Journal of Urology (2023)
-
A Novel Quantitative Electrocardiography Strategy Reveals the Electroinhibitory Effect of Tamoxifen on the Mouse Heart
Journal of Cardiovascular Translational Research (2023)
-
Effects of Postural Resonance on Skin Sympathetic Nerve Activity and Blood Pressure: A Pilot Study Evaluating Vascular Tone Baroreflex Stimulation Through Biofeedback
Applied Psychophysiology and Biofeedback (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.