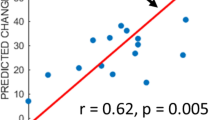
Abstract
Neuroimaging has radically improved our understanding of how speech and language abilities map to the brain in normal and impaired participants, including the diverse, graded variations observed in post-stroke aphasia. A handful of studies have begun to explore the reverse inference: creating brain-to-behaviour prediction models. In this study, we explored the effect of three critical parameters on model performance: (1) brain partitions as predictive features, (2) combination of multimodal neuroimaging and (3) type of machine learning algorithms. We explored the influence of these factors while predicting four principal dimensions of language and cognition variation in post-stroke aphasia. Across all four behavioural dimensions, we consistently found that prediction models derived from diffusion-weighted data did not improve performance over models using structural measures extracted from T1 scans. Our results provide a set of principles to guide future work aiming to predict outcomes in neurological patients from brain imaging data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The conditions of our ethical approval do not permit the public archiving of anonymized study data. The anonymized data necessary for reproducing the results in this article can be requested from the corresponding authors.
Code availability
The computer code that supports the findings of this study is available from the corresponding author on reasonable request.
References
Adamson, J., Beswick, A. & Ebrahim, S. Is stroke the most common cause of disability? J. Stroke Cerebrovasc. Dis. 13, 171–177 (2004).
Berthier, M. L. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging 22, 163–182 (2005).
Engelter, S. T. et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 37, 1379–1384 (2006).
Halai, A. D., Woollams, A. M. & Lambon Ralph, M. A. Predicting the pattern and severity of chronic post-stroke language deficits from functionally-partitioned structural lesions. NeuroImage Clin. 19, 1–13 (2018).
Hope, T. M. H., Leff, A. P. & Price, C. J. Predicting language outcomes after stroke: is structural disconnection a useful predictor? NeuroImage Clin. 19, 22–29 (2018).
Hope, T. M. H., Seghier, M. L., Leff, A. P. & Price, C. J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage Clin. 22, 424–433 (2013).
Hope, T. M. H. et al. Comparing language outcomes in monolingual and bilingual stroke patients. Brain 138, 1070–1083 (2015).
Pustina, D. et al. Enhanced estimations of post-stroke aphasia severity using stacked multimodal predictions. Hum. Brain Mapp. 38, 5603–5615 (2017).
Yourganov, G., Fridriksson, J., Rorden, C., Gleichgerrcht, E. & Bonilha, L. Multivariate connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J. Neurosci. 36, 6668–6679 (2016).
Yourganov, G., Smith, K. G., Fridriksson, J. & Rorden, C. Predicting aphasia type from brain damage measured with structural MRI. Cortex 73, 203–215 (2015).
Godefroy, O., Dubois, C., Debachy, B., Leclerc, M. & Kreisler, A. Vascular aphasias: main characteristics of patients hospitalized in acute stroke units. Stroke 33, 702–705 (2002).
Kasselimis, D. S., Simos, P. G., Peppas, C., Evdokimidis, I. & Potagas, C. The unbridged gap between clinical diagnosis and contemporary research on aphasia: a short discussion on the validity and clinical utility of taxonomic categories. Brain Lang. 164, 63–67 (2017).
Poeppel, D., Emmorey, K., Hickok, G. & Pylkkänen, L. Towards a new neurobiology of language. J. Neurosci. 32, 14125–14131 (2012).
Schwartz, M. F. What the classical aphasia categories can’t do for us, and why. Brain Lang. 21, 3–8 (1984).
Butler, R. A., Lambon Ralph, M. A. & Woollams, A. M. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain 137, 3248–2366 (2014).
Halai, A. D., Woollams, A. M. & Lambon Ralph, M. A. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex 86, 275–289 (2017).
Lacey, E. H., Skipper-Kallal, L. M., Xing, S., Fama, M. E. & Turkeltaub, P. E. Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabil. Neural Repair 31, 442–450 (2017).
Mirman, D. et al. Neural organization of spoken language revealed by lesion–symptom mapping. Nat. Commun. 6, 6762 (2015).
Mirman, D., Zhang, Y., Wang, Z., Coslett, H. B. & Schwartz, M. F. The ins and outs of meaning: behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia 76, 208–219 (2015).
Patterson, K. & Lambon Ralph, M. A. Selective disorders of reading? Curr. Opin. Neurobiol. 9, 235–239 (1999).
Seidenberg, M. S. & McClelland, J. L. A distributed, developmental model of word recognition and naming. Psychol. Rev. 96, 523–568 (1989).
Ueno, T., Saito, S., Rogers, T. T. & Lambon Ralph, M. A. Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal–ventral language pathways. Neuron 72, 385–396 (2011).
Ueno, T. & Lambon Ralph, M. A. The roles of the ‘ventral’ semantic and ‘dorsal’ pathways in conduite d’approche: a neuroanatomically-constrained computational modeling investigation. Front. Hum. Neurosci. 7, 422 (2013).
Shen, X., Tokoglu, F., Papademetris, X. & Constable, R. T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 82, 403–415 (2013).
Craddock, R. C., James, G. A., Holtzheimer, P. E., Hu, X. P. & Mayberg, H. S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928 (2012).
Saur, D. et al. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain 133, 1252–1264 (2010).
Michotey, P., Moskow, N. P. & Salamon, G. in Radiology of the Skull and Brain (eds Newton, T. H. & Poots, D. G.) 1471–1478 (Mosby, 1974).
Zhao, Y., Halai, A. D. & Lambon Ralph, M. A. Evaluating the granularity and statistical structure of lesions and behaviour in post-stroke aphasia. Preprint at bioRxiv https://doi.org/10.1101/802595 (2019).
Basilakos, A. et al. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front. Hum. Neurosci. 8, 845 (2014).
Eggert, G.H. Wernicke's Works on Aphasia: A Sourcebook and Review (Mouton de Gruyter, 1977).
Kinoshita, M. et al. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct. Funct. 220, 3399–3412 (2015).
Duffau, H., Gatignol, P., Mandonnet, E., Capelle, L. & Taillandier, L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with grade II glioma in the left dominant hemisphere. J. Neurosurg. 109, 461–471 (2008).
Marebwa, B. K. et al. Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Sci. Rep. 7, 8188 (2017).
Geller, J., Thye, M. & Mirman, D. Estimating effects of graded white matter damage and binary tract disconnection on post-stroke language impairment. Neuroimage 189, 248–257 (2019).
Hope, T. M. H., Seghier, M. L., Prejawa, S., Leff, A. P. & Price, C. J. Distinguishing the effect of lesion load from tract disconnection in the arcuate and uncinate fasciculi. Neuroimage 125, 1169–1173 (2016).
Marchina, S. et al. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 42, 2251–2256 (2011).
Abraham, A. et al. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 8, 14 (2014).
Grotegerd, D. et al. MANIA—a pattern classification toolbox for neuroimaging data. Neuroinformatics 12, 471–486 (2014).
Hanke, M. et al. PyMVPA: a Python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics 7, 37–53 (2009).
Hanke, M. et al. PyMVPA: a unifying approach to the analysis of neuroscientific data. Front. Neuroinform. 3, 3 (2009).
Hebart, M. N. & Baker, C. I. Deconstructing multivariate decoding for the study of brain function. Neuroimage 180, 4–18 (2018).
Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008).
LaConte, S., Strother, S., Cherkassky, V., Anderson, J. & Hu, X. Support vector machines for temporal classification of block design fMRI data. Neuroimage 26, 317–329 (2005).
Oosterhof, N. N., Connolly, A. C. & Haxby, J. V. CoSMoMVPA: multi-modal multivariate pattern analysis of neuroimaging data in MATLAB/GNU Octave. Front. Neuroinform. 10, 27 (2016).
Pereira, F. & Botvinick, M. Information mapping with pattern classifiers: a comparative study. Neuroimage 56, 476–496 (2011).
Schrouff, J. et al. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics 11, 19–37 (2013).
Huang, J. & Zhang, T. The benefit of group sparsity. Ann. Stat. 38, 1978–2004 (2010).
Filippone, M. et al. Probabilistic prediction of neurological disorders with a statistical assessment of neuroimaging data modalities. Ann. Appl. Stat. 6, 1883–1905 (2012).
Haufe, S. et al. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 87, 96–110 (2014).
Weichwald, S. et al. Causal interpretation rules for encoding and decoding models in neuroimaging. Neuroimage 110, 48–59 (2015).
Schrouff, J., Mourão-Miranda, J., Phillips, C. & Parvizi, J. Decoding intracranial EEG data with multiple kernel learning method. J. Neurosci. Methods 261, 19–28 (2016).
Schrouff, J. et al. Embedding anatomical or functional knowledge in whole-brain multiple kernel learning models. Neuroinformatics 16, 117–143 (2018).
Penny, W., Friston, K., Ashburner, J., Kiebel, S. & Nichols, T., eds. Statistical Parametric Mapping: The Analysis of Functional Brain Images (Academic Press, 2007).
Jeffreys, H. The Theory of Probability (Oxford Univ. Press, 1961).
Goodglass, H. & Kaplan, E. The Assessment of Aphasia and Related Disorders: Revised (Lea & Febiger, 1972).
Kertesz, A. Western Aphasia Battery (Grune & Stratton, 1982).
Kümmerer, D. et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain 136, 619–629 (2013).
Geschwind, N. The organization of language and the brain. Science 170, 940–944 (1970).
Hickok, G. & Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402 (2007).
Lichtheim, L. in Broca’s Region (eds Grodzinsky, Y. & Amunts, K.) 318–334 (Oxford Univ. Press, 2009).
Catani, M. & Ffytche, D. H. The rises and falls of disconnection syndromes. Brain 128, 2224–2239 (2005).
Staffaroni, A. M. et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain 142, 443–459 (2019).
Alyahya, R. S. W., Halai, A. D., Conroy, P. & Lambon Ralph, M. A. Noun and verb processing in aphasia: behavioural profiles and neural correlates. NeuroImage Clin. 18, 215–230 (2018).
Alyahya, R. S. W., Halai, A. D., Conroy, P. & Lambon Ralph, M. A. The behavioural patterns and neural correlates of concrete and abstract verb processing in aphasia: a novel verb semantic battery. NeuroImage Clin. 17, 811–825 (2018).
Conroy, P., Sotiropoulou Drosopoulou, C., Humphreys, G. F., Halai, A. D. & Lambon Ralph, M. A. Time for a quick word? The striking benefits of training speed and accuracy of word retrieval in post-stroke aphasia. Brain 141, 1815–1827 (2018).
Woollams, A. M., Halai, A. D. & Lambon Ralph, M. A. Mapping the intersection of language and reading: the neural bases of the primary systems hypothesis. Brain Struct. Funct. 223, 3769–3786 (2018).
Halai, A. D., Woollams, A. M. & Lambon Ralph, M. A. Triangulation of language–cognitive impairments, naming errors and their neural bases post-stroke. NeuroImage Clin. 17, 465–473 (2018).
Tochadse, M., Halai, A. D., Lambon Ralph, M. A. & Abel, S. Unification of behavioural, computational and neural accounts of word production errors in post-stroke aphasia. NeuroImage Clin. 18, 952–962 (2018).
Schumacher, R., Halai, A. D. & Lambon Ralph, M. A. Assessing and mapping language, attention and executive multidimensional deficits in stroke aphasia. Brain 142, 3202–3216 (2019).
Seghier, M. L., Ramlackhansingh, A., Crinion, J., Leff, A. P. & Price, C. J. Lesion identification using unified segmentation–normalisation models and fuzzy clustering. Neuroimage 41, 1253–1266 (2008).
Kay, J., Lesser, R. & Coltheart, M. Psycholinguistic Assessments of Language Processing in Aphasia: PALPA: Aphasiology (Psychology Press, 1992).
Bozeat, S., Lambon Ralph, M. A., Patterson, K., Garrard, P. & Hodges, J. R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 38, 1207–1215 (2000).
Kaplan, E., Goodglass, H. & Weintraub, S. The Boston Naming Test (Lea & Febinger, 1983).
Jefferies, E., Patterson, K., Jones, R. W. & Lambon Ralph, M. A. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology 23, 492–499 (2009).
Swinburn, K., Baker, G. & Howard, D. CAT: Comprehensive Aphasia Test (Psychology Press, 2005).
Wechsler, D. A. Wechsler Memory Scale—Revised (Psychological Corporation, 1987).
Burgess, P. W. & Shallice, T. The Hayling and Brixton Tests (Pearson Clinical, 1997).
Raven, J. C. Advanced Progressive Matrices, Set II (H. K. Lewis, 1962).
Ballabio, D. A MATLAB toolbox for principal component analysis and unsupervised exploration of data structure. Chemometr. Intell. Lab. Syst. 149, 1–9 (2015).
Bro, R., Kjeldahl, K., Smilde, A. K. & Kiers, H. A. L. Cross-validation of component models: a critical look at current methods. Anal. Bioanal. Chem. 390, 1241–1251 (2008).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851 (2005).
Wilke, M., de Haan, B., Juenger, H. & Karnath, H. O. Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. Neuroimage 56, 2038–2046 (2011).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556–572 (2016).
Behrens, T. E. J., Berg, H. J., Jbabdi, S., Rushworth, M. F. S. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155 (2007).
Behrens, T. E. J. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Bozzali, M. et al. Anatomical connectivity mapping: a new tool to assess brain disconnection in Alzheimer’s disease. Neuroimage 54, 2045–2051 (2011).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
Hastie, T., Tibshirani, R. & Friedman, J. Elements of Statistical Learning (Springer, 2009); https://doi.org/10.1007/978-0-387-84858-7
Tipping, M. E. Sparse Bayesian learning and the relevance vector machine. J. Mach. Learn. Res. 1, 211–244 (2001).
Rasmussen, C. E. & Williams, C. K. I. Gaussian Processes for Machine Learning (MIT Press, 2006).
Bach, F. R., Lanckriet, G. R. G. & Jordan, M. I. Multiple kernel learning, conic duality, and the SMO algorithm. in Proc. Twenty-First International Conference on Machine Learning (ICML 2004) https://doi.org/10.1145/1015330.1015424 (Association for Computing Machinery, 2004).
Rakotomamonjy, A., Bach, F. R., Canu, S. & Grandvalet, Y. SimpleMKL. J. Mach. Learn. Res. 9, 2491–2521 (2008).
Morey, R. D. et al. BayesFactor: Computation of Bayes Factors for Common Designs. https://cran.r-project.org/web/packages/BayesFactor/index.html (CRAN, 2018).
Fritz, C. O., Morris, P. E. & Richler, J. J. Effect size estimates: current use, calculations, and interpretation. J. Exp. Psychol. Gen. 141, 2–18 (2012).
Rosenthal, R. in The Handbook of Research Synthesis (eds Cooper, H. & Hedges, L. V.) 231–244, (Sage, 1994).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015).
Acknowledgements
We thank all the patients, families, carers and community support groups for their continued, enthusiastic support of our research programme. This research was supported by grants from The Rosetrees Trust (no. A1699) and ERC (GAP: 670428 - BRAIN2MIND_NEUROCOMP). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.D.H., A.M.W. and M.A.L.R. conceived and designed the experiment. A.D.H. collected and analysed the data. A.D.H., A.M.W. and M.A.LR. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Marike Schiffer.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Supplementary Results 1.
Rights and permissions
About this article
Cite this article
Halai, A.D., Woollams, A.M. & Lambon Ralph, M.A. Investigating the effect of changing parameters when building prediction models for post-stroke aphasia. Nat Hum Behav 4, 725–735 (2020). https://doi.org/10.1038/s41562-020-0854-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-020-0854-5
This article is cited by
-
An optimal hybrid AI-ResNet for accurate severity detection and classification of patients with aphasia disorder
Signal, Image and Video Processing (2023)
-
3D printed triboelectric nanogenerator as self-powered human-machine interactive sensor for breathing-based language expression
Nano Research (2022)
-
Mapping lesion, structural disconnection, and functional disconnection to symptoms in semantic aphasia
Brain Structure and Function (2022)
-
Auditory beat perception is related to speech output fluency in post-stroke aphasia
Scientific Reports (2021)
-
Predicting post-stroke aphasia from brain imaging
Nature Human Behaviour (2020)