Abstract
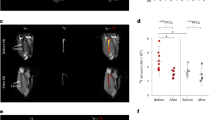
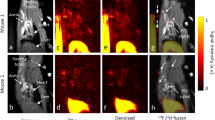
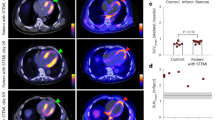
Ischaemic heart disease evokes a complex immune response. However, tools to track the systemic behaviour and dynamics of leukocytes non-invasively in vivo are lacking. Here, we present a multimodal hot-spot imaging approach using an innovative high-density lipoprotein-derived nanotracer with a perfluoro-crown ether payload (19F-HDL) to allow myeloid cell tracking by 19F magnetic resonance imaging. The 19F-HDL nanotracer can additionally be labelled with zirconium-89 and fluorophores to detect myeloid cells by in vivo positron emission tomography imaging and optical modalities, respectively. Using our nanotracer in atherosclerotic mice with myocardial infarction, we observed rapid myeloid cell egress from the spleen and bone marrow by in vivo 19F-HDL magnetic resonance imaging. Concurrently, using ex vivo techniques, we showed that circulating pro-inflammatory myeloid cells accumulated in atherosclerotic plaques and at the myocardial infarct site. Our multimodality imaging approach is a valuable addition to the immunology toolbox, enabling the study of complex myeloid cell behaviour dynamically.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 24, 711–720 (2018).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Tawakol, A. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–845 (2017).
Thackeray, J. T. et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J. Am. Coll. Cardiol. 71, 263–275 (2018).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Roth, S. et al. Brain-released alarmins and stress response synergize in accelerating atherosclerosis progression after stroke. Sci. Transl. Med. 10, eaao1313 (2018).
Pérez-Medina, C. et al. A modular labeling strategy for in vivo PET and near-infrared fluorescence imaging of nanoparticle tumor targeting. J. Nucl. Med. 55, 1706–1711 (2014).
Tang, J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016).
Pérez-Medina, C. et al. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc. Imaging 9, 950–961 (2016).
Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L. & Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14, 133–140 (1994).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Panizzi, P. et al. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J. Am. Coll. Cardiol. 55, 1629–1638 (2010).
von Elverfeldt, D. et al. Dual-contrast molecular imaging allows noninvasive characterization of myocardial ischemia/reperfusion injury after coronary vessel occlusion in mice by magnetic resonance imaging. Circulation 130, 676–687 (2014).
Heusch, G. et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383, 1933–1943 (2014).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Soehnlein, O. Multiple roles for neutrophils in atherosclerosis. Circ. Res. 110, 875–888 (2012).
Holland, J. P., Sheh, Y. & Lewis, J. S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 36, 729–739 (2009).
Plump, A. S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992).
Tarnavski, O. et al. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genomics 16, 349–360 (2004).
Acknowledgements
This work was supported by De Drie Lichten Foundation in the Netherlands (M.L.S.); American Heart Association grant nos. 17PRE33660729 (M.L.S.) and 16SDG3139000 (C.P.M.); German Research Foundation grant nos. MA 7059/1 (A.M.) and HO 5953/1-1 (M.N.); National Heart, Lung, and Blood Institute grant nos. HL096576, HL117829, HL139598 and HL128264; the MGH Research Scholar Award (M.N. and F.K.S.); Dutch Applied and Engineering Sciences (TTW) grant no. 14716 (G.J.S); National Institutes of Health (NIH) grants: R01HL143814, R01HL144072, R01HL135878 and P01HL131478 (Z.A.F.); R01HL144072, R01CA220234 and P01HL131478 (W.J.M.M.); and the Netherlands Organisation for Scientific Research (NWO) grants: Vidi 91713324 and Vici 91818622 (W.J.M.M.).
Author information
Authors and Affiliations
Contributions
M.L.S. coordinated the experimental planning and execution. W.J.M.M. conceptualized and designed the study. A.J.P.T., B.L.S.-G., C.P.-M. and E.D.K. developed, synthesized, characterized and labelled the nanotracers. M.L.S., A.E.M., M.M.T.L., J.C.V., Y.C.T., A.M., E.D.K., N.A.T.S., A.M.S., H.G., C.F., R.S.O., E.M.L., F.F. and E.C. conducted the in vivo experiments. C.C., Z.A.F. and G.J.S. developed the imaging protocols. M.N. and F.K.S. developed the flow cytometry protocols and provided immunological insights. R.M.D., R.D. and L.Z. contributed expertise on cardiovascular disease mouse models. S.H. and T.R. designed nanotracer labelling strategies. M.L.S. and W.J.M.M. wrote the manuscript and M.L.S. produced the figures for initial submission. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Jeff Bulte, Christian Schulz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Rights and permissions
About this article
Cite this article
Senders, M.L., Meerwaldt, A.E., van Leent, M.M.T. et al. Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging. Nat. Nanotechnol. 15, 398–405 (2020). https://doi.org/10.1038/s41565-020-0642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0642-4
This article is cited by
-
Nanomedicines for cardiovascular disease
Nature Cardiovascular Research (2023)
-
Biodegradable polyphosphoester micelles act as both background-free 31P magnetic resonance imaging agents and drug nanocarriers
Nature Communications (2023)
-
PET/MR imaging of inflammation in atherosclerosis
Nature Biomedical Engineering (2022)
-
Systematically evaluating DOTATATE and FDG as PET immuno-imaging tracers of cardiovascular inflammation
Scientific Reports (2022)
-
In vivo optical molecular imaging of inflammation and immunity
Journal of Molecular Medicine (2021)