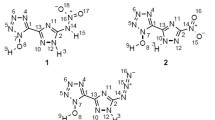
Abstract
Density functional theory calculations were performed to explore four octahedral energetic complexes including [CoCl2 (ANQ)2], [Co (ANQ)2(H2O)2]2+, [CuCl2 (ANQ)2], and [Cu(NO3)2 (ANQ)2], (ANQ = amino nitroguanidine). In this work, an attempt has been made to present useful structural data in order to investigate and predict the explosive properties of these complexes. In this regard, interaction energy (IE), natural bond orbital (NBO), atoms in a molecule (AIM) as well as the three-dimensional Hirshfeld surface analysis and the two-dimensional fingerprint plots, charge transfers, HUMO-LUMO gap, oxygen balance (%OB) amounts, and molecular electrostatic potential (MEP) maps were utilized to assign intermolecular interactions, bond lengths, the nature of metal-ligand bonds, and energies in subject compounds. The results reveal that among the five applied levels of theory, interaction energies obtaining from M06-2X/Def2TZVP were in excellent compliance with the experiments. Additionally, the N⋯O interaction, oxygen balance, density, and HOMO–LUMO gap were the most contributing factors in assigning sensitivity and detonation properties. In general, the sensitivity and detonation properties are increased in the following order: ANQ < complex1 < complex3 < complex2 < complex4.

Graphical abstract








Similar content being viewed by others
References
Badgujar D, Talawar M, Asthana S, Mahulikar P (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151:289–305
Klapötke TM (2007) High energy density materials. Springer, Berlin Heidelberg
Wang SW, Yang L, Feng JL, Wu BD, Zhang JG, Zhang TL, Zhou ZN (2011) Synthesis, crystal structure, thermal decomposition, and sensitive properties of two novel energetic cadmium (II) complexes based on 4-amino-1, 2, 4-triazole. Z Anorg Allg Chem 637:2215–2222
Jeong K, Jeon Y, Kwon S (2017) Assessment of various DFT, DFT-D, and MP2 methods for studying FOX-7 detonation properties. J Mol Model 23:250
Tao G-H, Parrish DA, Shreeve JM (2012) Nitrogen-rich 5-(1-methylhydrazinyl) tetrazole and its copper and silver complexes. Inorg Chem 51:5305–5312
Hofmann A (1866) XXIV.—on the synthesis of guanidine. J Chem Soc 19:249–255
Berlinck RG, Burtoloso ACB, Kossuga MH (2008) The chemistry and biology of organic guanidine derivatives. Nat Prod Rep 25:919–954
Bondu S, Genta-Jouve G, Leirόs M, Vale C, Guigonis J-M, Botana LM, Thomas OP (2012) Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. RSC Adv 2:2828–2835
Sączewski F, Balewski Ł (2013) Biological activities of guanidine compounds, 2008–2012 update. Expert Opin Ther Pat 23:965–995
Ishikawa T (2010) Guanidine chemistry. Chem Pharm Bull 58:1555–1564
Alonso-Moreno C, Antinolo A, Carrillo-Hermosilla F, Otero A (2014) Guanidines: from classical approaches to efficient catalytic syntheses. Chem Soc Rev 43:3406–3425
Garlets ZJ, Silvi M, Wolfe JP (2016) Synthesis of cyclic guanidines via silver-catalyzed intramolecular alkene hydroamination reactions of N-allylguanidines. Org Lett 18:2331–2334
Klapötke TM, Mieskes F, Stierstorfer J, Weyrauther M (2016) Studies on energetic salts based on (2, 4, 6-trinitrophenyl) guanidine. Propellants Explos Pyrotech 41:217–222
Dippold A, Klapötke TM, Martin FA (2011) Synthesis and characterization of bis (triaminoguanidinium) 5, 5′-dinitrimino-3, 3′-azo-1H-1, 2, 4-triazolate–a novel insensitive energetic material. Z Anorg Allg Chem 637:1181–1193
Klapötke TM (2017) Chemistry of high-energy materials. Walter de Gruyter GmbH & Co KG, Berlin
Metelkina E, Novikova T, Berdonosova S, Berdonosov DY (2005) 2-Nitroguanidine derivatives: IX. Reaction of 1-amino-2-nitroguanidine with oxalic acid as a method of synthesis of 3 (5)-nitroamino-1, 2, 4-triazole-5 (3)-carboxylic acid and 5, 5′-bi (3-nitroamino-1, 2, 4-triazole) salts. Russ J Org Chem 41:440–443
Fischer N, Joas M, Klapötke TM, Stierstorfer J (2013) Transition metal complexes of 3-amino-1-nitroguanidine as laser ignitible primary explosives: structures and properties. Inorg Chem 52:13791–13802
Lothrop WC, Handrick GR (1949) The relationship between performance and constitution of pure organic explosive compounds. Chem Rev 44:419–445
Zhang J, He C, Parrish DA, Shreeve JM (2013) Nitramines with varying sensitivities: functionalized dipyrazolyl-N-nitromethanamines as energetic materials. Chem Eur J 19:8929–8936
Fischer D, Klapötke TM, Stierstorfer J (2014) Synthesis and characterization of diaminobisfuroxane. Eur J Inorg Chem 2014:5808–5811
Guo T, Wang Z, Tang W, Wang W, Bi F, Wang B, Zhou Z, Meng Z, Ge Z (2018) A good balance between the energy density and sensitivity from assembly of bis (dinitromethyl) and bis (fluorodinitromethyl) with a single furazan ring. J Anal Appl Pyrolysis 134:218–230
Bader R (1990) Atoms in molecule. A Quantum Theory
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Fukui K, Yonezawa T, Shingu H (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20:722–725
Parr RG, Zhou Z (1993) Absolute hardness: unifying concept for identifying shells and subshells in nuclei, atoms, molecules, and metallic clusters. Acc Chem Res 26:256–258
Ayers PW, Parr RG, Pearson RG (2006) Elucidating the hard/soft acid/base principle: a perspective based on half-reactions. J Chem Phys 124:194107
Aihara J-i (1999) Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A 103:7487–7495
Aihara J-i (2000) Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys Chem Chem Phys 2:3121–3125
Wolff S, Grimwood D, McKinnon J, Turner M, Jayatilaka D, Spackman M (2012) Crystal explorer. The University of Western Australia Perth, Crawley
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4:378–392
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J Chem Soc Perkin Trans 2:S1–S19
Bitzer RS, Visentin LC, Hörner M, Nascimento MA, Filgueiras CA (2017) On the molecular and supramolecular properties of N, N′-disubstituted iminoisoindolines: synthesis, spectroscopy, X-ray structure and Hirshfeld surface analyses, and DFT calculations of two (E)-N, N′-bis (aryl) iminoisoindolines (aryl= 2-tert-butylphenyl or perfluorophenyl). J Mol Struct 1130:165–173
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993). J Comput Chem 14:1347–1363
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2006) Density functional for spectroscopy: no long-range self-interaction error, good performance for Rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J Phys Chem A 110:13126–13130
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Ehlers A, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler K, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc-Cu, Y-Ag and La-Au. Chem Phys Lett 208:111–114
Höllwarth A, Böhme M, Dapprich S, Ehlers A, Gobbi A, Jonas V, Köhler K, Stegmann R, Veldkamp A, Frenking G (1993) A set of d-polarization functions for pseudo-potential basis sets of the main group elements Al-Bi and f-type polarization functions for Zn, Cd, Hg. Chem Phys Lett 208:237–240
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Glendening E, Reed A, Carpenter J, Weinhold F (1998), NBO Version 3.1, There is no corresponding record for this reference
Biegler-könig FW, Bader RF, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comput Chem 3:317–328
Acknowledgments
We would like to thank anonymous reviewers for helpful comments and our colleagues from the Malek-Ashtar University of Technology Shahin Shahr, who provide valuable insights.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Roohzadeh, R., Mahdavi, M. Prediction of explosive properties of newly synthesized amino nitroguanidine-based energetic complexes via density functional theory. J Mol Model 26, 104 (2020). https://doi.org/10.1007/s00894-020-04377-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04377-6