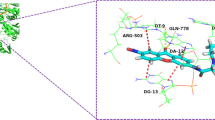
Abstract
Series of novel N-benzyl derivatives of 6-aminoflavone (9a–n) were synthesized and evaluated for anticancer and topoisomerase II enzyme inhibition activity. All the synthesized compounds were screened for in vitro anticancer activity against human breast cancer cell line (MCF-7) and human lung cancer cell line (A-549). Among the synthesized compounds, 9f and 9g were found to be the most potent anticancer agents against human breast cancer cell line (MCF-7) with IC50 values of 9.35 µM and 9.58 µM, respectively. Compounds 9b, 9c and 9n exhibited promising anticancer activity against human lung cancer cell line (A-549) with 43.71%, 46.48% and 44.26% inhibition at the highest concentration of 10 µM, respectively. Compounds 9c, 9f and 9g have ability to inhibit the topoisomerase II enzyme. Compound 9f showed most potent topoisomerase II enzyme inhibition activity with IC50 value of 12.11 µM. Further, these compounds have a high potential to be developed as a promising topoisomerase II inhibitors.



Similar content being viewed by others
References
Verma AK, Pratap R (2010) The biological potential of flavones. Nat Prod Rep 27:1571–1593. https://doi.org/10.1039/C004698C
Moon MJ, Lee SK, Lee JW et al (2006) Synthesis and structure–activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg Med Chem 14:237–246. https://doi.org/10.1016/j.bmc.2005.08.008
Zhai S, Senderowicz AM, Sausville EA, Figg WD (2002) Flavopiridol, a novel cyclin-dependent kinase inhibitor, in clinical development. Ann Pharmacother 36:905–911. https://doi.org/10.1345/aph.1A162
Panaro NJ, Popescu NC, Harris SR, Thorgeirsson UP (1999) Flavone acetic acid induces a G2/M cell cycle arrest in mammary carcinoma cells. Br J Cancer 80:1905–1911. https://doi.org/10.1038/sj.bjc.6690619
Alexandrakis M, Singh L, Boucher W, Letourneau R, Theoflopoulos P, Theoharides TC (1999) Differential effect of flavonoids on inhibition of secretion and accumulation of secretory granules in rat basophilic leukemia cells. Int J Immunopharmacol 21:379–390. https://doi.org/10.1016/S0192-0561(99)00018-1
Bernard FX, Sable S, Cameron B, Provost J, Desnottes JF, Crouzet J, Blanche F (1997) Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Chemother 41:992–998. https://doi.org/10.1128/AAC.41.5.992
Mittra B, Saha A, Roy Chowdhury A et al (2000) Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated Kinetoplast DNA cleavage leading to apoptosis. Mol Med 6:527–541. https://doi.org/10.1007/BF03401792
Bhosle MR, Wahul DB, Bondle GM, Sarkate A, Tiwari SV (2018) An efficient multicomponent synthesis and in vitro anticancer activity of dihydropyranochromene and chromenopyrimidine-2,5-diones. Syn Commun 48:2046–2060. https://doi.org/10.1080/00397911.2018.1480042
Gobbi S, Cavalli A, Ram A et al (2006) Lead optimization providing a series of flavone derivatives as potent nonsteroidal inhibitors of the cytochrome P450 aromatase enzyme. J Med Chem 49:4777–4780. https://doi.org/10.1021/jm060186y
Alworth WL, Dang CC, Ching LM, Viswanathan T (1980) Stimulation of mammalian epoxide hydrase activity by flavones. Xenobiotica 10:395–400. https://doi.org/10.3109/00498258009033774
Beutler JA, Cardellina Ii JH, Lin CM, Hamel E, Craggand GM, Boyd MR (1993) Centaureidin, a cytotoxic flavone from Polymnia fruticosa, inhibits tubulin polymerization. Bioorg Med Chem Lett 3:581–584. https://doi.org/10.1016/S0960-894X(01)81233-6
Lichius JJ, Thoison O, Montagnac A et al (1994) Antimitotic and cytotoxic flavonols from Zieridium pseudobtusifolium and Acronychia porter. J Nat Prod 57:1012–1016. https://doi.org/10.1021/np50109a024
Lin CM, Singh SB, Chu PS et al (1988) Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure–activity study. Mol Pharmacol 34:200–208
Singh M, Kaur M, Silakari O (2014) Flavones: an important scaffold for medicinal chemistry. Eur J Med Chem 84:206–239. https://doi.org/10.1016/j.ejmech.2014.07.013
Konya K, Pajtas D, Kiss-Szikszai A, Patonay T (2015) Buchwald–Hartwig reactions of monohaloflavones. Eur J Org Chem 4:828–839. https://doi.org/10.1002/ejoc.201403108
Pajtás D, Patonay T, Kónya K (2016) Synthesis of 8-bromoflavone and its Buchwald–Hartwig reaction with amines. Synthesis 48:97–102. https://doi.org/10.1055/s-0035-1560325
Wiegand R, Wu J, Sha X, LoRusso P, Heath E, Li J (2009) Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate aminoflavone (NSC 686288) in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 877:1460–1464. https://doi.org/10.1016/j.jchromb.2009.03.015
Patra N, De U, Kang JA et al (2011) A novel epoxypropoxy flavonoid derivative and topoisomerase II inhibitor, MHY336, induces apoptosis in prostate cancer cells. Eur J Pharmacol 658:98–107. https://doi.org/10.1016/j.ejphar.2011.02.015
Lazaro ML, Willmore E, Austin CA (2010) The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat Res 696:41–47. https://doi.org/10.1016/j.mrgentox.2009.12.010
Cassady JM, Baird WM, Chang CJ (1990) Natural products as a source of potential cancer chemotherapeutic and chemopreventive agents. J Nat Prod 53:23–41. https://doi.org/10.1021/np50067a003
Kim MY, Na Y, Vankayalapati H, Guzman MG, Hurley LH (2003) Design, synthesis, and evaluation of psorospermin/quinobenzoxazine hybrids as structurally novel antitumor agents. J Med Chem 46:2958–2972. https://doi.org/10.1021/jm030096i
Singh S, Baviskar AT, Jain V et al (2013) 3-Formylchromone based topoisomerase IIα inhibitors: discovery of potent leads. Med Chem Commun 4:1257–1266. https://doi.org/10.1039/C3MD00125C
Simon L, Srinivasan KK, Mallikarjuna Rao C et al (2015) Synthesis and evaluation of anti-cancer activity of some 6-aminoflavones. Int J Pharm Chem 5:240–246. https://doi.org/10.7439/ijpc.v5i7.2220
Thorat NM, Kote SR, Thopate SR (2014) An efficient and green synthesis of flavones using natural organic acids as promoter under solvent-free condition. Lett Org Chem 11:601–605. https://doi.org/10.2174/157017861108140613163214
Wendorff TJ, Schmidt BH, Heslop P, Austin CA, Berger JM (2012) The structure of DNA-bound human topoisomerase II alpha: conformational mechanisms for coordinating inter-subunit interactions with DNA cleavage. J Mol Biol 424:109–124. https://doi.org/10.1016/j.jmb.2012.07.014
Friesner RA, Murphy RB, Repasky MP et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem 49:6177–6196. https://doi.org/10.1021/jm051256o
Tiwari SV, Seijas JA, Vazquez-Tato MP, Sarkate AP, Lokwani DK, Nikalje AG (2016) Ultrasound mediated one-pot, three component synthesis, docking and ADME prediction of novel 5-amino-2-(4-chlorophenyl)-7-substituted phenyl-8,8a-dihydro-7h-(1,3,4)thiadiazolo(3,2-α)pyrimidine-6-carbonitrile derivatives as anticancer agents. Molecules 21:894. https://doi.org/10.3390/molecules21080894
Tiwari SV, Seijas JA, Vazquez-Tato MP, Sarkate AP, Karnik KS, Nikalje AG (2017) Facile synthesis of novel coumarin derivatives, antimicrobial analysis, enzyme assay, docking study, ADMET prediction and toxicity study. Molecules 22:1172. https://doi.org/10.3390/molecules22071172
Doherty W, Adler N, Knox A, Nolan D, McGouran J, Nikalje AP, Lokwani D, Sarkate A, Evans P (2017) Synthesis and evaluation of 1,2,3-triazole-containing vinyl and allyl sulfones as anti-trypanosomal agents. Eur J Org Chem 1:175–185. https://doi.org/10.1002/ejoc.201601221
Dofe VS, Sarkate AP, Azad R, Gill CH (2017) Novel quinoline-based oxadiazole derivatives induce G2/M arrest and apoptosis in human breast cancer MCF-7 cell line. Res Chem Int 43:7331–7345. https://doi.org/10.1007/s11164-017-3078-1
Ibrahim MK, Taghour MS, Metwaly AM et al (2018) Design, synthesis, molecular modeling and anti-proliferative evaluation of novel quinoxaline derivatives as potential DNA intercalators and topoisomerase II inhibitors. Eur J Med Chem 155:117–134. https://doi.org/10.1016/j.ejmech.2018.06.004
Acknowledgements
We thank Dr. D. K. Mhaske, Dr. L. R. Patil (Maharaja Jivajirao Shinde College, Shrigonda) and Dr. R. J. Barnabas (Ahmednagar College, Ahmednagar) for their constant support. NMT acknowledges the financial support by BCUD, SPPU, Pune (Grant No: 13SCI 000031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thorat, N.M., Sarkate, A.P., Lokwani, D.K. et al. N-Benzylation of 6-aminoflavone by reductive amination and efficient access to some novel anticancer agents via topoisomerase II inhibition. Mol Divers 25, 937–948 (2021). https://doi.org/10.1007/s11030-020-10079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10079-1