Abstract
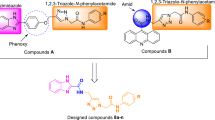
Fourteen novel 4,5-diphenyl-imidazol-1,2,3-triazole hybrids 8a–n were synthesized with good yields by performing click reaction between the 4,5-diphenyl-2-(prop-2-yn-1-ylthio)-1H-imidazole and various benzyl azides. The synthesized compounds 8a–n were evaluated against yeast α-glucosidase, and all these compounds exhibited excellent inhibitory activity (IC50 values in the range of 85.6 ± 0.4–231.4 ± 1.0 μM), even much more potent than standard drug acarbose (IC50 = 750.0 μM). Among them, 4,5-diphenyl-imidazol-1,2,3-triazoles possessing 2-chloro and 2-bromo-benzyl moieties (compounds 8g and 8i) demonstrated the most potent inhibitory activities toward α-glucosidase. The kinetic study of the compound 8g revealed that this compound inhibited α-glucosidase in a competitive mode. Furthermore, docking calculations of these compounds were performed to predict the interaction mode of the synthesized compounds in the active site of α-glucosidase.
Graphic abstract
A novel series of 4,5-diphenyl-imidazol-1,2,3-triazole hybrids 8a–n was synthesized with good yields by performing click reaction between the 4,5-diphenyl-2-(prop-2-yn-1-ylthio)-1Himidazole and various benzyl azides. The synthesized compounds 8a–n were evaluated against yeast α-glucosidase and all these compounds exhibited excellent inhibitory activity (IC50 values in the range of 85.6 ± 0.4-231.4 ± 1.0 μM), even much more potent than standard drug acarbose (IC50 = 750.0 μM).







Similar content being viewed by others
References
Schmidt DD, Frommer W, Junge B, Müller L, Wingender W, Truscheit E, Schäfer D (1997) α-Glucosidase inhibitors. Naturwissenschaften 64:535–536. https://doi.org/10.1007/BF00483561
Matsuo T, Odaka H, Ikeda H (1992) Effect of an intestinal disaccharidase inhibitor (AO-128) on obesity and diabetes. Am J Clin Nutr 55:314S–317S. https://doi.org/10.1093/ajcn/55.1.314s
Scott LJ, Spencer CM (2000) Miglitol Drugs 59:521–549. https://doi.org/10.2165/00003495-200059030-00012
Asano N, Oseki K, Tomioka E, Kizu H, Matsui K (1994) N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr Res 259:243–255. https://doi.org/10.1016/0008-6215(94)84060-1
Hollander P (1992) Safety profile of acarbose, an α-glucosidase inhibitor. Drugs 44:47–53. https://doi.org/10.2165/00003495-199200443-00007
Adisakwattana S, Sookkongwaree K, Roengsumran S, Petsom A, Ngamrojnavanich N, Chavasiri W, Deesamer S, Yibchok-anun S (2004) Structure–activity relationships of trans-cinnamic acid derivatives on α-glucosidase inhibition. Bioorg Med Chem Lett 14:2893–2896. https://doi.org/10.1016/j.bmcl.2004.03.037
Sou S, Mayumi S, Takahashi H, Yamasaki R, Kadoya S, Sodeoka M, Hashimoto Y (2000) Novel α-glucosidase inhibitors with a tetrachlorophthalimide skeleton. Bioorg Med Chem Lett 10:1081–1084. https://doi.org/10.1016/S0960-894X(00)00161-X
Abboud JL, Foces-Foces C, Notario R, Trifonov RE, Volovodenko AP, Ostrovskii VA, Alkorta I, Elguero J (2001) Basicity of N-H-and N-methyl-1,2,3-triazoles in the gas phase, in solution, and in the solid state—an experimental and theoretical study. Eur J Org Chem 16:3013–3024. https://doi.org/10.1002/1099-0690(200108)2001:16%3c3013:AID-EJOC3013%3e3.0.CO;2-Y
Vatmurge NS, Hazra BG, Pore VS, Shirazi F, Chavan PS, Deshpande MV (2008) Synthesis and antimicrobial activity of β-lactam–bile acid conjugates linked via triazole. Bioorg Med Chem Lett 18:2043–2047. https://doi.org/10.1016/j.bmcl.2008.01.102
Thirumurugan P, Matosiuk D, Jozwiak K (2013) Click chemistry for drug development and diverse chemical–biology applications. Chem Rev 113:4905–4979. https://doi.org/10.1021/cr200409f
Aufort M, Herscovici J, Bouhours P, Moreau N, Girard C (2008) Synthesis and antibiotic activity of a small molecules library of 1,2,3-triazole derivatives. Bioorg Med Chem Lett 18:1195–1198. https://doi.org/10.1016/j.bmcl.2007.11.111
Kumar A, Ahmad I, Chhikara BS, Tiwari R, Mandal D, Parang K (2011) Synthesis of 3-phenylpyrazolopyrimidine-1,2,3-triazole conjugates and evaluation of their Src kinase inhibitory and anticancer activities. Bioorg Med Chem Lett 21:1342–1346. https://doi.org/10.1016/j.bmcl.2011.01.047
Lima-Neto RG, Cavalcante NN, Srivastava RM, Mendonça Junior FJ, Wanderley AG, Neves RP, dos Anjos JV (2012) Synthesis of 1, 2, 3-triazole derivatives and in vitro antifungal evaluation on Candida strains. Molecules 17:5882–5892. https://doi.org/10.3390/molecules17055882
Shanmugavelan P, Nagarajan S, Sathishkumar M, Ponnuswamy A, Yogeeswari P, Sriram D (2011) Efficient synthesis and in vitro antitubercular activity of 1,2,3-triazoles as inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem Lett 21:7273–7276. https://doi.org/10.1016/j.bmcl.2011.10.048
Mohammadi-Khanaposhtani M, Mahdavi M, Saeedi M, Sabourian R, Safavi M, Khanavi M, Foroumadi A, Shafiee A, Akbarzadeh T (2015) Design, synthesis, biological evaluation, and docking study of acetylcholinesterase inhibitors: new acridone-1,2,4-oxadiazole-1,2,3-triazole hybrids. Chem Biol Drug Des 86:1425–1432. https://doi.org/10.1111/cbdd.12609
Brik A, Alexandratos J, Lin YC, Elder JH, Olson AJ, Wlodawer A, Goodsell DS, Wong CH (2005) 1,2,3-Triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors. ChemBioChem 6:1167–1169. https://doi.org/10.1002/cbic.200500101
Iqbal S, Khan MA, Javaid K, Sadiq R, Fazal-ur-Rehman S, Choudhary MI, Basha FZ (2017) New carbazole linked 1,2,3-triazoles as highly potent non-sugar α-glucosidase inhibitors. Bioorg Chem 74:72–81. https://doi.org/10.1016/j.bioorg.2017.07.006
Wang G, Peng Z, Wang J, Li X, Li J (2017) Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur J Med Chem 125:423–429. https://doi.org/10.1016/j.ejmech.2016.09.067
Saeedi M, Mohammadi-Khanaposhtani M, Pourrabia P, Razzaghi N, Ghadimi R, Imanparast S, Faramarzi MA, Bandarian F, Esfahani EN, Safavi M, Rastegar H (2019) Design and synthesis of novel quinazolinone-1,2,3-triazole hybrids as new anti-diabetic agents: in vitro α-glucosidase inhibition, kinetic, and docking study. Bioorg Chem 83:161–169. https://doi.org/10.1016/j.bioorg.2018.10.023
Yar M, Bajda M, Shahzad S, Ullah N, Gilani MA, Ashraf M, Rauf A, Shaukat A (2015) Organocatalyzed solvent free an efficient novel synthesis of 2,4,5-trisubstituted imidazoles for α-glucosidase inhibition to treat diabetes. Bioorg Chem 58:65–71. https://doi.org/10.1016/j.bioorg.2014.11.006
Wang G, Peng Z, Wang J, Li J, Li X (2016) Synthesis and biological evaluation of novel 2,4,5-triarylimidazole-1,2,3-triazole derivatives via click chemistry as α-glucosidase inhibitors. Bioorg Med Chem Lett 26:5719–5723. https://doi.org/10.1016/j.bmcl.2016.10.057
Adib M, Peytam F, Shourgeshty R, Mohammadi-Khanaposhtani M, Jahani M, Imanparast S, Faramarzi MA, Larijani B, Moghadamnia AA, Esfahani EN, Bandarian F (2019) Design and synthesis of new fused carbazole-imidazole derivatives as anti-diabetic agents: in vitro α-glucosidase inhibition, kinetic, and in silico studies. Bioorg Med Chem Lett 29:713–718. https://doi.org/10.1016/j.bmcl.2019.01.012
Mohammadi-Khanaposhtani M, Yahyavi H, Barzegaric E, Imanparast S, Heravi MM, Ali Faramarzi M, Foroumadi A, Adibi H, Larijani B, Mahdavi M (2018) New biscoumarin derivatives as potent α-glucosidase inhibitors: synthesis, biological evaluation, kinetic analysis, and docking study. Polycycl Aromat Comp 5:1–2. https://doi.org/10.1080/10406638.2018.1509359
Adib M, Peytam F, Rahmanian-Jazi M, Mohammadi-Khanaposhtani M, Mahernia S, Bijanzadeh HR, Jahani M, Imanparast S, Faramarzi MA, Mahdavi M, Larijani B (2018) Design, synthesis and in vitro α-glucosidase inhibition of novel coumarin-pyridines as potent antidiabetic agents. New J Chem 42:17268–17278. https://doi.org/10.1039/C8NJ02495B
Adib M, Peytam F, Rahmanian-Jazi M, Mahernia S, Bijanzadeh HR, Jahani M, Mohammadi-Khanaposhtani M, Imanparast S, Faramarzi MA, Mahdavi M, Larijani B (2018) New 6-amino-pyrido [2,3-d] pyrimidine-2,4-diones as novel agents to treat type 2 diabetes: a simple and efficient synthesis, α-glucosidase inhibition, molecular modeling and kinetic study. Eur J Med Chem 155:353–363. https://doi.org/10.1016/j.ejmech.2018.05.046
Seul, South Corea: Bioinformatics and Molecular Design Research Center; 2004. PreADMET program. http://preadmet.bmdrc.org
Maduskuie TP, Wilde RG, Billheimer JT, Cromley DA, Germain S, Gillies PJ, Higley CA, Johnson AL, Pennev P, Shimshick EJ, Wexler RR (1995) Design, synthesis, and structure-activity relationship studies for a new imidazole series of J774 macrophage specific acyl-CoA: cholesterol acyltransferase (ACAT) inhibitors. J Med Chem 38:1067–1083. https://doi.org/10.1021/jm00007a004
Veltri L, Mancuso R, Altomare A, Gabriele B (2015) Divergent multicomponent tandem palladium-catalyzed aminocarbonylation-cyclization approaches to functionalized imidazothiazinones and imidazothiazoles. ChemCatChem 7:2206–2213. https://doi.org/10.1002/cctc.201500213
Acknowledgements
This project was financially supported by the National Institute for Medical Research Development (NIMAD) (the Grant Number: 977073).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asgari, M.S., Mohammadi-Khanaposhtani, M., Sharafi, Z. et al. Design and synthesis of 4,5-diphenyl-imidazol-1,2,3-triazole hybrids as new anti-diabetic agents: in vitro α-glucosidase inhibition, kinetic and docking studies. Mol Divers 25, 877–888 (2021). https://doi.org/10.1007/s11030-020-10072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10072-8