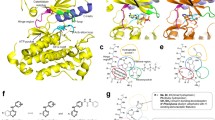
Abstract
Receptor tyrosine kinase c-Met is an important antitumor drug target. Triazolotriazine analogues 2–10 were prepared efficiently and evaluated the enzymatic and cellular c-Met activities. Brief structure–activity relationships of triazolotriazine core and CF2-quinoline part were investigated, leading to the discovery of compound 8 with nanomolar enzymatic c-Met activity, and subnanomolar MKN45 and EBC-1 cellular potencies. The proposed binding model of 8 and c-Met unraveled that two canonical hydrogen bonds and a π–π stacking interaction formed between the inhibitor and the ATP binding site of c-Met kinase domain, which accounted for its potent c-Met activities.
Graphic abstract





Similar content being viewed by others
References
Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251:802–804
Gherardi E, Youles ME, Miguel RN, Blundell TL, Iamele L, Gough J, Bandyopadhyay A, Hartmann G, Butler PJG (2003) Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 100:12039–12044
Peters S, Adjei AA (2012) MET: a promising anticancer therapeutic target. Natl Rev Clin Oncol 9:314–326
Blumenschein GR Jr, Mills GB, Gonzalez-Angulo AM (2012) Targeting the hepatocyte growth factor c-Met axis in cancer therapy. J Clin Oncol 30:3287–3296
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12:89–103
Goyal L, Muzumdar MD, Zhu AX (2013) Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res 19:2310–2318
Rong S, Segal S, Anver M, Resau JH, Vande Woude GF (1994) Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 91:4731–4735
Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery JP, Jouanneau J (1994) Creation of a hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene 9:1091–1099
Jeffers M, Rong S, Anver M, Vande Woude GF (1996) Autocrine hepatocyte growth factor/scatter factor signaling induces transformation and the invasive/metastatic phenotype in C127 cells. Oncogene 13:853–861
Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G (1997) Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 94:701–706
Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, Merlino G (1998) c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res 58:5157–5167
Aguirre Ghiso JA, Alonso DF, Farías EF, Gomez DE, de Kier Joffè EB (1999) Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur J Biochem 263:295–304
Parr C, Watkins G, Mansel RE, Jiang WG (2004) The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res 10(1 Pt 1):202–211
Cui JJ (2014) Targeting receptor tyrosine kinase MET in cancer: small molecule inhibitors and clinical progress. J Med Chem 57:4427–4453
Liu X, Yao W, Newton RC, Scherle PA (2008) Targeting the c-Met signaling pathway for cancer therapy. Expert Opin Invest Drugs 17:997–1011
Cui JJ (2007) Inhibitors targeting hepatocyte growth factor receptor and their potential therapeutic applications. Expert Opin Ther Pat 17:1035–1045
Parikh PK, Ghate MD (2018) Recent advances in the discovery of small molecule c-Met kinase inhibitors. Eur J Med Chem 143:1103–1138
Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H, Yu Y, Wang H, Xu S, Zhou F, Su X, Yin X, Xie L, Wang L, Qing W, Jiao L, Su W, Wang QM (2015) Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol 9:323–333
Jia H, Dai G, Weng J, Zhang Z, Wang Q, Zhou F, Jiao L, Cui Y, Ren Y, Fan S, Zhou J, Qing W, Gu Y, Wang J, Sai Y, Su W (2014) Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem 57:7577–7589
Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, Jia L, Meng J, Funk L, Botrous I, McTigue M, Grodsky N, Ryan K, Padrique E, Alton G, Timofeevski S, Yamazaki S, Li Q, Zou H, Christensen J, Mroczkowski B, Bender S, Kania RS, Edwards MP (2011) Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal–epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 54:6342–6363
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, Laird AD, Engst S, Lee L, Lesch J, Chou Y-C, Joly AH (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298–2308
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Co-hen RB, Mehra R, Pfister DG, Cohen EEW, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Müller T, Ratain MJ, Salgia R (2011) Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29:2660–2666
Eathiraj S, Palma R, Volckova E, Hirschi M, France DS, Ashwell MA, Chan TC (2011) Discovery of a novel mode of protein kinase inhibition characterized by the mechanism of inhibition of human mesenchymal-epithelial transition factor (c-Met) protein autophosphorylation by ARQ 197. J Biol Chem 286:20666–20676
Yan M, Wang H, Wang Q, Zhang Z, Zhang C (2016) Allo steric inhibition of c-Met kinase in sub-microsecond molecular dynamics simulations induced by its inhibitor, tivantinib. Phys Chem Chem Phys 18:10367–10374
Zhan Z, Peng X, Liu Q, Chen F, Ji Y, Yao S, Xi Y, Lin Y, Chen T, Xu Y, Ai J, Geng M, Duan W (2016) Discovery of 6-(difluoro(6-(4-fluorophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-3-yl)methyl)quinoline as a highly potent and selective c-Met inhibitor. Eur J Med Chem 116:239–251
Owusu BY, Thomas S, Venukadasula P, Han Z, Janetka JW, Galemmo RA Jr, Klampfer L (2017) Targeting the tumor-promoting microenvironment in MET-amplified NSCLC cells with a novel inhibitor of pro-HGF activation. Oncotarget 8:63014–63025
Liu X, Wang Q, Yang G, Marando C, Koblish HK, Hall LM, Fridman JS, Behshad E, Wynn R, Li Y, Boer J, Diamond S, He C, Xu M, Zhuo J, Yao W, Newton RC, Scherle PA (2011) A novel kinase inhibitor, INCB28060, blocks c-MET dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res 17:7127–7138
Cui JJ, McTigue M, Nambu M, Tran-Dubé M, Pairish M, Shen H, Jia L, Cheng H, Hoffman J, Le P, Jalaie M, Goetz GH, Ryan K, Grodsky N, Deng YL, Parker M, Timofeevski S, Murray BW, Yamazaki S, Aguirre S, Li Q, Zou H, Christensen J (2012) Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-MET) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1-yl)ethanol (PF-04217903) for the treatment of cancer. J Med Chem 55:8091–8109
Acknowledgements
We thank Shanghai Sailing Program (No. 17YF1423300), the Youth Innovation Promotion Association of CAS (No. 2018324), National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program”, China (No. 2018ZX09711002-011-016), “Strategic Priority Research Program” of the Chinese Academy of Sciences (No. XDA12020228), and the National Natural Science Foundation of China (No. 21702220) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Y., Peng, X., Ji, Y. et al. Synthesis of triazolotriazine derivatives as c-Met inhibitors. Mol Divers 25, 839–846 (2021). https://doi.org/10.1007/s11030-020-10067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10067-5