Abstract
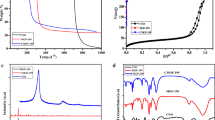
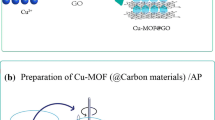
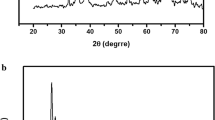
In this work, we report the synthesis of a Cu (II) based metal–organic frameworks (HKUST-1) by mechanochemical method and metal-oxide–carbon nanocomposite (CuO@C) as thermolysis products. The prepared HKUST-1 and composite were characterized by elemental CHNS/O microanalysis, scanning electron microscopy equipped with energy dispersive X-ray spectroscopy (SEM–EDX), transmission electron microscopy (TEM), ultraviolet–visible spectroscopy (UV–vis), thermogravimetric analyses (TGA), fourier-transform infrared spectroscopy (FTIR), powder X-ray diffraction (PXRD) and Brunauer–Emmett–Teller (BET) analysis. The TEM analysis, confirmed the CuO nanoparticles in the porous carbon matrix. The synthesized compounds were further investigated for adsorption of Cd (II) from aqueous solution. The composite exhibited a high cadmium uptake of 132.6 mg g−1 compared to the parent HKUST-1, and fast kinetics with a kinetic rate constant k2 of 0.031 g mg−1 min−1, which is greater than some existing adsorbents for cadmium adsorption from aqueous solution under similar condition. The cadmium adsorption on CuO@C composite showed pH, temperature and time dependence to the prepared HKUST-1, as well as greater surface area and pore volume. The CuO@C composite can be readily recycled and regenerated without significant loss of the cadmium adsorption capacity.









Similar content being viewed by others
References
Y. Shen (2015). J. Mater. Chem. A. 3, 13188.
W. Yao, C. Shen, and Y. Lu (2013). Compos. Sci. Technol. 87, 13.
S. Flandrois and B. Simon (1999). Carbon N. Y. 37, 180.
Z. Hu, M. P. Srinivasan, and Y. Ni (2000). Adv. Mater. 12, 65.
X. Tong, Y. Qin, and X. Guo (2012). Small 8, 3395.
X. Sun (2003). J. Dispers. Sci. Technol. 24, 567.
T.-H. Kim, K.-B. Lee, and J.-W. Choi (2013). Biomaterials 34, 8670.
S. Miyanaga, H. Yasuda, and A. Hiwara (1990). J. Macromol. Sci. 27, 1361.
G. Férey (2008). Chem. Soc. Rev. 37, 214.
S. H. Jhung, N. A. Khan, and Z. Hasan (2012). CrystEngComm 14, 7109.
J.-J. Chen, Y.-T. Chen, and D. S. Raja (2015). Sci. Technol. Adv. Mater. 16, 54203.
J. Liu, L. Chen, and H. Cui (2014). Chem. Soc. Rev. 43, 6061.
W. Chaikittisilp, K. Ariga, and Y. Yamauchi (2013). J. Mater. Chem. A 1, 19.
J.-K. Sun and Q. Xu (2014). Energy Environ. Sci. 7, 2100.
H. Yue, Z. Shi, and Q. Wang (2014). ACS Appl. Mater. Interfaces 6, 17074.
X. Yan, N. Lu, and B. Fan (2015). CrystEngComm 17, 6433.
Z. Feng, S. Zhu, and D. R. Martins de Godoi (2012). Anal. Chem. 84, 3770.
M. P. Waalkes (2000). J. Inorg. Biochem. 79, 244.
M. Xu, P. Hadi, G. Chen, and G. McKay (2014). J. Hazard. Mater. 273, 123.
M. E. Clares, M. G. Guerrero, and M. García-González (2015). Int. J. Environ. Sci. Technol. 12, 1798.
Y. Ge, D. Xiao, Z. Li, and X. Cui (2014). J. Mater. Chem. A 2, 2145.
S. S. Gupta and K. G. Bhattacharyya (2012). Phys. Chem. Chem. Phys. 14, 6723.
Y. Wang, G. Ye, and H. Chen (2015). J. Mater. Chem. A 3, 15298.
H.-T. Fan, J.-X. Liu, and H. Yao (2014). Ind. Eng. Chem. Res. 53, 378.
A. Pichon, A. Lazuen-Garay, and S. L. James (2006). CrystEngComm 8, 214.
A. El-Trass, H. ElShamy, I. El-Mehasseb, and M. El-Kemary (2012). Appl. Surf. Sci. 258, 3001.
J.-J. Chen, Y.-T. Chen, and D. S. Raja (2015). Materials 8, 5347.
R. Qadeer and S. Akhtar (2005). Turk. J. Chem. 29, 100.
M. Ajmal, R. A. K. Rao, R. Ahmad, and J. Ahmad (2000). J. Hazard. Mater. 79, 131.
F. Ge, M.-M. Li, H. Ye, and B.-X. Zhao (2012). J. Hazard. Mater. 211, 372.
F. A. B. Silva and F. L. Pissetti (2014). J. Colloid Interfaces Sci. 416, 100.
I. Langmuir (1918). J. Am. Chem. Soc. 40, 1403.
Z. Hasan, N. A. Khan, and S. H. Jhung (2016). Chem. Eng. J. 284, 1413.
A. Dubey, A. Mishra, and S. Singhal (2014). Int. J. Environ. Sci. Technol. 11, 1050.
X. Weng, S. Lin, Y. Zhong, and Z. Chen (2013). Chem. Eng. J. 229, 34.
Acknowledgements
We are grateful to the School of Chemistry, University of KwaZulu-Natal, Durban, South Africa for spectroscopy analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oseghale, C.O., Elemike, E.E., Ayi, A.A. et al. Thermolytic Conversion of Copper (II) Based Coordination Polymer into Copper Oxide–Carbon Nanocomposite for Selective Removal of Cd (II) from Aqueous Solution. J Clust Sci 32, 319–326 (2021). https://doi.org/10.1007/s10876-020-01790-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01790-y