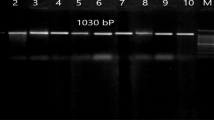
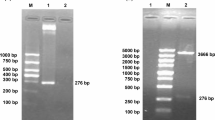
Abstract
Pasteurella multocida is the main cause of haemorrhagic septicaemia (HS) outbreak in livestock, such as cattle and buffaloes. Conventional vaccines such as alum-precipitated or oil-adjuvant broth bacterins were injected subcutaneously to provide protection against HS. However, the immunity developed is only for short term and needed to be administered frequently. In our previous study, a short gene fragment from Pasteurella multocida serotype B was obtained via shotgun cloning technique and later was cloned into bacterial expression system. pQE32-ABA392 was found to possess immunogenic activity towards HS when tested in vivo in rat model. In this study, the targeted gene fragment of ABA392 was sub-cloned into a DNA expression vector pVAX1 and named as pVAX1-ABA392. The new recombinant vaccine was stable and expressed on mammalian cell lines. Serum sample collected from a group of vaccinated rats for ELISA test shows that the antibody in immunized rats was present at high titer and can be tested as a vaccine candidate with challenge in further studies. This successful recombinant vaccine is immunogenic and potentially could be used as vaccine in future against HS.



Similar content being viewed by others
References
Wijewardana, T. G. (1992). Haemorrhagic septicaemia. Reviews in Medical Microbiology,3, 59–63.
Abdullah, F. F. J., Osman, A. Y., Adamu, L., Yusof, M. S. M., Omar, A. R., Saharee, A. A., Haron, A. W., Abdullah, R., & Zamri-Saad, M. (2013). Polymerase chain reaction detection of Pasteurella multocida type B: 2 in mice following oral inoculation. Asian Journal of Animal and Veterinary Advances, 8, 493–501.
Abubakar, M. S., & Zamri-Saad, M. (2011). Clinico-pathological changes in buffalo calves following oral exposure to Pasteurella multocida B:2. Basic Applied Pathol,4, 130–135.
De Alwis, M. C. L. (1999). Haemorrhagic septicaemia ACIAR Monograph No. Canberra: Australian Centre for International Agricultural Research.
Harper, M., Boyce, J. D., & Adler, B. (2006). Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiology Letters,265, 1–10.
Benkirane, A., & De Alwis, M. C. L. (2002). Haemorrhagic septicaemia, its significance, prevention and control in Asia. Veterinary Medicine Czechoslovakia,47(8), 234–240.
Marza, D. A., Jesse, F. F. A., Ihsan, M. A., et al. (2016). Involvement of the nervous system following experimental infection with Pasteurella multocida B:2 in buffalo (Bubalus bubalis): A clinicopathological study. Microbe Pathology,93, 111–119.
Annas, S., Zamri-Saad, M., Jesse, F. F. A., & Zunita, Z. (2015). Comparative clinic pathological changes in buffalo and cattle following infection by Pasteurella multocida B:2. Microbe Pathology,88, 94–102.
Zamri-Saad, M. (2013). Haemorrhagic Septicaemia of cattle and buffaloes in Asia Malaysia. Malaysia: Universit Putra Malaysia Press.
Kamarudin, M. I. (2005). Haemorrhagic septicaemia: Eradication is a possibility. In: Proceedings of the Regional Symposium on Haemorrhagic Septicaemia, December 1–2, pp. 12–15.
Ferreira, T. S. P. F., Moreno, L. Z., Felizardo, M. R., Gobbi, D. D. S., Filsner, P. H. L. N., Gomes, V. T. M., Moreno, M., Moreno, A. M. (2016) Pheno-and genotypic characterization of Pasteurella multocida isolated from cats, dogs and rabbits from Brazil. Comparative Immunology, Microbiology and Infectious Diseases, 45, 48–52.
Thomas, J. (1972). The control of hemorrhagic septicaemia in West Malaysia. Tropical Animal Health and Production,4, 95–101.
Al-Hasani, K., Boyce, J., McCarl, V. P., Bottomley, S., Wilkie, I., & Adler, B. (2007). Identification of novel immunogens in Pasteurella multocida. Microbial Cell Factories,6(1), 3.
Bosch, M., Garrido, M. E., Roza, A. M. P., Badiola, I., Barbe, J., & Llagostera, M. (2004). Pasteurella multocida contain multiple immnugenic haemin- and haemoglobin-binding proteins. Veterinary Microbiology,99, 103–111.
Salmah, I. (2000). Molecular characterization of virulence-determinant of recombinant clone ABA392 from P. multocida PMB202 of serotype B animal isolate. Dissertation, University of Malaya, Kuala Lumpur
Ismail, S. (2004). Application of DNA technology in determining the virulence genes responsible for haemorrhagic septicaemia of Pasteurella multocida serotype B by using shotgun cloning. Asian Journal of Information Technology (AJIT), 3(12), 1221–1224.
Salmah, I. (1997). Molecular studies of Pasteurella multocida animal isolates. Dissertation, University of Malaya, Kuala Lumpur
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed.). New York: Cold Spring Harbor Laboratory Press.
Close, T. I., & Rodriguez, R. L. (1982). Construction and characterisation of the chloramphenicol-resistance gene cartridge: A new approach to the transcriptional mapping of extrachromosomal elements. Gene,20(305), 316.
Hussaini, J., Nazmul, M. H. M., Mahmood, A. A., & Salmah, I. (2012). Recombinant Clone ABA392 protects laboratory animals from Pasteurella multocida Serotype B. Journal of Veterinary Advances,2(2), 114–119.
Einarsdottir, T., Gunnarsson, E., Sigurdardottir, O. G., Jorundsson, E., Fridriksdottir, V., Thorarinsdottir, G. E., et al. (2016). Variability of Pasteurella multocida isolated from Icelandic sheep and detection of the toxA gene. Journal of Medical Microbiology,65(9), 897–904.
Corrongean, M. (1902). Bovine Pasteurella in the Malay Peninsula. The Veterinary Journal,55, 321–327.
Joseph, P. G. (1979). Hemorrhagic septicaemia in Peninsular Malaysia. Kajian Veterinar,11, 65–79.
Zulperi, D. M. (2008). Development of an Avirulent Pasteurella Multocida B: 2 by Disruption of the ABA392 DNA Fragment. Dissertation, Universiti Putra Malaysia, Selangor.
Chandrasekaran, S., Kennett, L., Yeap, P. C., Muniandy, N., Rani, B., & Mukkur, T. K. S. (1994). Characterization of immune response and duration of protection in buffaloes immunized with haemorrhagic septicaemia vaccines. Veterinary Microbiology, 41, 213–219.
Tabatabaei, M., Moazzeni Jula, G. R., Jabbari, A. R., & Esmailzadesh, M. (2007). Pathogenicity and immunogenicity of native and mutant strains of Pasteurella multocida, the causative agents of haemorrhagic septicemia. Iranian Journal of Veterinary Research,8, 40–44.
Montgomery, D. L., & Prather, K. J. (2006). Design of plasmid DNA constructs for vaccines. DNA Vaccines,1, 11–22.
Williams, J. A., Carnes, A. E., & Hodgson, C. P. (2009). Plasmid DNA vaccine vector design: Impact on efficacy, safety and upstream production. Biotechnology Advances,27(4), 353–370.
Wigler, M., Silverstein, S., Lee, L. S., Pellicer, A., Cheng, Y., & Axel, R. (1977). Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell,11, 223–232.
Zhang, Y., Ma, C. H., Liu, H., Zhang, X. M., & Sun, W. S. (2007). pVAX1 plasmid vector-mediated gene transfer of soluble TRAIL suppresses human hepatocellular carcinoma growth in nude mice. Acta Biochimica Polonica,54(2), 307.
Garcia, J. L., Innes, E. A., & Katzer, F. (2014). Current progress toward vaccines against Toxoplasma gondii. Vaccine.,4(1), 23–28.
Williams, J. (2013). Vector design for improved DNA vaccine efficacy, safety and production. Vaccines,1(3), 225–249.
Singh, S., Singh, V. P., Cheema, P. S., Sandey, M., Ranjan, R., Gupta, S. K., et al. (2011). Immune response to DNA vaccine expressing transferrin binding protein a gene of Pasteurella multocida. Brazilian Journal of Microbiology,42(2), 750–760.
Kowalczyk, D. W., & Ertl, H. C. J. (1999). Immune responses to DNA vaccines. Cellular and Molecular Life Sciences,55, 751–770.
Skinner, M. A., Buddle, B. M., Wedlock, D. N., Keen, D., Lisle, G. W., & Tascon, R. E. (2003). A DNA prime-BCG boost vaccination strategy in cattle induces protection against bovis tuberculosis. Infection and Immunity,71, 4901–4907.
Ahmad, T. A., Rammah, S. S., Sheweita, S. A., & Haroun, M. (2014). Development of immunization trials against Pasteruella multocida. Vaccine,32, 909–917.
Hussaini, J. (2009). Analysis of recombinant DNA fragment derived from Pasteurella multocida genome with haemorrhagic and immunogenic properties: Towards a vaccine development. Dissertation, University Teknologi MARA.
Register, K. B., Sacco, R. E., & Brockmeier, S. L. (2007). Immune response in mice and swineto DNA vaccines derived from the Pasteurella multocida toxin gene. Vaccine,25, 6118–6128.
Gong, Q., Cheng, M., & Niu, M. (2011). Out membrane protein DNA vaccines for protec-tive immunity against virulent avian Pasteurella multocida in chickens. Procedia Environmental Sciences,8, 723–729.
Acknowledgement
This research was supported by Postgraduate Research Grant (PPP) PG237-2014B Grant under University of Malaya. The authors are grateful to all Molecular Bacteriology Toxicology members for their laboratory assistance during this study.
Author information
Authors and Affiliations
Contributions
SI, SC, RDV, KTL and CWKS conceived and planned the research. SC and KTL performed the research. SC, NK and SI contributed to the analyzed of the result. HAR and NNR provided critical feedback and contributed new methods. SC and SI took the lead in writing manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chelliah, S., Velappan, R.D., Lim, K.T. et al. Potential DNA Vaccine for Haemorrhagic Septiceamia Disease. Mol Biotechnol 62, 289–296 (2020). https://doi.org/10.1007/s12033-020-00244-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00244-0