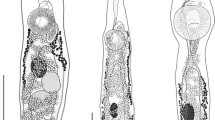
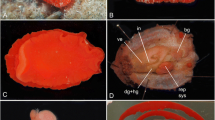
Abstract
Three new monorchiid trematodes are reported from fishes of the families Nemipteridae and Pomacanthidae; two species are described from the southern Great Barrier Reef and the third from off Moorea, French Polynesia. Allobacciger polynesiensis sp. nov. is described from Centropyge flavissima (Cuvier), Allobacciger brevicirrus sp. nov. from Scolopsis bilineata (Bloch) and Scolopsis margaritifera (Cuvier), and Allobacciger annulatus sp. nov. from Centropyge tibicen (Cuvier), Centropyge vroliki (Bleeker) and Centropyge bicolor (Bloch). The three species most closely resemble the type-species, Allobacciger macrorchis Hafeezullah & Siddiqi, 1970, as well as Allobacciger centropygis Machida & Uchida, 2001, differing primarily in the morphology of the terminal genitalia and excretory vesicle. With the introduction of these new species, the most recent generic concept does not accurately reflect all species in the genus, and we provide a revised generic diagnosis to accommodate the new taxa. Complete ITS2 rDNA, partial 28S rDNA, partial 18S rDNA and partial cox1 mtDNA sequence data were generated for the three new species; all markers distinguish the three species reported here unambiguously. Phylogenetic analyses of the new sequence data show that the three new species of Allobacciger Hafeezullah & Siddiqi, 1970 form a well-supported clade.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available in GenBank repository, https://www.ncbi.nlm.nih.gov/nucleotide. GenBank accession numbers are listed in Table 1.
References
Ankenbrand MJ, Keller A, Wolf M, Schultz J, Förster F (2015) ITS2 Database V: twice as much. Mol Biol Evol 32:3030–3032
Atopkin DM, Besprozvannykh VV, Ngo HD, Van Ha N, Van Tang N, Ermolenko AV, Beloded AY (2017) Morphometric and molecular data of the two digenean species Lasiotocus lizae Liu, 2002 (Monorchiidae) and Paucivitellosus vietnamensis sp. n. (Bivesiculidae) from mullet fish in Tonkin Bay, Vietnam. J Helminthol 91:346–355
Barnhart MC, Powell EC (1979) Lissorchis kritskyi sp. n. (Digenea: Lissorchiidae) from the River Carpsucker, Carpiodes carpio (Rafinesque). Proc Helminthol Soc Wash 46:47–51
Bartoli P, Bray RA (2004) Ancylocoelium typicum Nicoll, 1912 (Digenea: Monorchiidae), a poorly known parasite of Trachurus spp. (Teleostei: Carangidae) from the western Mediterranean and north-eastern Atlantic, and observations on its taxonomic position. Syst Parasitol 58:23–29
Besprozvannykh VV, Ermolenko AV, Atopkin DM (2012) The life cycle of Asymphylodora percotti sp. n. (Trematoda: Lissorchiidae) in the Russian Southern Far East. Parasitol Int 61:235–241
Boaden AE, Kingsford MJ (2012) Diel behaviour and trophic ecology of Scolopsis bilineatus (Nemipteridae). Coral Reefs 31:871–883
Bray RA, Cribb TH (1996) Two Lepotrema Ozaki, 1932 species (Digenea: Lepocreadiidae) fro marine fishes from the southern Great Barrier Reef, Queensland, Australia. Syst Parasitol 35:111–117
Bray RA, Cribb TH, Barker SC (1993) The Lepocreadiidae (Digenea) of pomacentrid fishes (Perciformes) from Heron Island, Queesnland, Australia. Syst Parasitol 26:189–200
Bray RA, Cribb TH, Barker SC (1996) Cableia pudica n. sp. (Digenea: Acanthocolpidae) from monacanthid fishes of the southern Great Barrier Reef, Australia. Parasite 3:49–54
Bray RA, Cribb TH, Cutmore SC (2018a) Lepocreadiidae Odhner, 1905 and Aephnidiogenidae Yamaguti, 1934 (Digenea: Lepocreadioidea) of fishes from Moreton Bay, Queensland, Australia, with the erection of a new family and genus. Syst Parasitol 95:479–498
Bray RA, Cutmore SC, Cribb TH (2018b) Lepotrema Ozaki, 1932 (Lepocreadiidae: Digenea) from Indo-Pacific fishes, with the description of eight new species, characterised by morphometric and molecular features. Syst Parasitol 95:693–741
Cremonte F, Kroeck MA, Martorelli SR (2001) A new monorchiid cercaria (Digenea) parasitising the purple clam Amiamtis purpurata (Bivalvia: Veneridae) in the Southwest Atlantic Ocean, with notes on its gonadal effect. Folia Parasitol 48:217–223
Cribb TH, Bray RA (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst Parasitol 76:1–7
Cribb TH, Anderson GR, Adlard RD, Bray RA (1998) A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Playtyhelminthes: Digenea). Int J Parasitol 28:1791–1795
Cribb TH, Anderson GR, Bray RA (1999) Faustulid trematodes (Digenea) from marine fishes of Australia. Syst Parasitol 44:119–138
Cribb TH, Wee NQ-X, Bray RA, Cutmore SC (2018) Monorchis lewisi n. sp. (Trematoda: Monorchiidae) from the surf bream, Acanthopagrus australis (Sparidae), in Moreton Bay, Australia. J Helminthol 92:100-108
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dove ADM, Cribb TH (1998) Two new genera, Provitellus and Ovipusillus, and four new species of Monorchiidae (Digenea) from carangid fishes of Queensland, Australia. Syst Parasitol 48:21–33
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Elwood HJ, Olson GJ, Sogin ML (1985) The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol 2:399–410
Frédérich B, Santini F, Konow N, Schnitzler J, Lecchini D, Alfaro ME (2017) Body shape convergence driven by small size optimum in marine angelfishes. Biol Lett 13:1–5
Gilardoni C, Carballo MC, Cremonte F (2013) The life cycle and geographical distribution of the monorchiid Proctotrema bartolii (Digenea) in the clam Darina solenoides from the Patagonian coast, Argentina. J Helminthol 87:392–399
Hafeezullah M, Siddiqi H (1970) Digenetic trematodes of marine fishes of India. Part II. Fellodistomatidae. J Parasitol 56:932–940
Hopkins SH (1941) New genera and species of the family Monorchiidae (Trematoda), with a discussion of the excretory system. J Parasitol 27:397–407
ICZN (2012) International Commission on Zoological Nomenclature: amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull Zool Nomencl 69:161–169
Kearse M et al (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Keller A, Schleicher T, Schultz J, Müller T, Dandekar T, Wolf M (2009) 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 430:50–57
Konow N, Bellwood DR (2011) Evolution of high trophic diversity based on limited functional disparity in the feeding apparatus of marine angelfishes (f. Pomacanthidae). PLoS One 6(9):e24113
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lakshmi TT, Madhavi R (2009) A new monorchiid trematode, Paramonorcheides selaris n. sp., from the carangid fish Selar crumenophthalmus in the Bay of Bengal off the Visakhapatnam coast of India. Syst Parasitol 73:135–139
Littlewood DTJ, Olson PD (2001) Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 262–278
Littlewood DTJ, Rohde K, Clough KA (1997) Parasite speciation within or between host species?—phylogenetic evidence from site-specific polystome monogeneans. Parasitology 27:1289–1297
Littlewood DTJ, Curini-Galletti M, Herniou EA (2000) The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol Phylogenet Evol 16:449–466
Liu S-f (2002) Lasiotocus lizae sp. n. (Digenea: Monorchiidae), a new trematode from marine fish in the Taiwan Straits, China. Folia Parasitol 49:218–220
Looss A (1907) Zur Kentnis der Distomenfamilie Hemiuridae. Zool Anz 31:585–620
Machida M (2005) Monorchiidae (Trematoda, Digenea) from fishes of Japanese and adjacent waters. Bull Natl Sci MusTokyo Ser A 31:123–136
Machida M, Uchida K (2001) Digenean trematodes from pomacanthid fishes of Japan and Palau. Bulletin of the National Science Museum Tokyo, Series A 27:217–227
Maddison WP, Maddison DR (2019) Mesquite: a modular system for evolutionary analysis. Version 3.6. http://mesquiteproject.org
Madhavi R (2008) Family Monorchiidae Odhner, 1911. In: Bray RA, Gibson DI, Jones A (eds) Keys to the Trematoda, vol 3. CAB International and Natural History Museum, Wallingford-London, pp 145–175
Manter HW, Pritchard MH (1961) Studies on digenetic trematodes of Hawaiian fishes: Family Monorchiidae and Haploporidae. J Parasitol 47:483–492
Martin SB, Cutmore SC, Cribb TH (2017) Revision of Neolebouria Gibson, 1976 (Digenea: Opecoelidae), with Trilobovarium n. g., for species infecting tropical and subtropical shallow-water fishes. Syst Parasitol 94:307–338
McNamara MKA, Cribb TH (2011) Taxonomy, host specificity and dietary implications of Hurleytrematoides (Digenea: Monorchiidae) from chaetodontid fishes on the Great Barrier Reef. Parasitol Int 60:255–269
McNamara MKA, Miller TL, Cribb TH (2014) Evidence for extensive cryptic speciation in trematodes of butterflyfishes (Chaetodontidae) of the tropical Indo-West Pacific. Int J Parasitol 44:37–48
Morgan JA, Blair D (1995) Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology 111:609–615
Monticelli FS (1893) Studii sui Trematodi endoparassiti. Primo contributo di osservazioni sui Distomidi. Zool Jahrb:1–229
Nahhas FM, Sey O, Nishimoto R (1998) Digenetic trematodes of marine fishes from the Kuwaiti Coast of the Arabian Gulf: families Pleorchiidae, Fellodistomidae, and Cryptogonimidae, with a description of two new species, Neoparacryptogonimus sphericus and Paracryptogonimus ramadani. J Helminthol Soc W 65:129–140
Nicoll W (1909) Studies on the structure and classification of the digenetic trematodes. Q J Microsc Sci 53:391–488
Nicoll W (1912) On two new trematode parasites from british food-fishes. Parasitology 5:197–202
Odhner T (1905) Die Trematoden des arktischen Gebietes. G. Fischer, Leipzig
Odhner T (1911) Zum natürlichen System der digenen Trematoden III. Zool Anz 38:97–117
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33:733–755
Ronquist F et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Searle EL, Cutmore SC, Cribb TH (2014) Monorchiid trematodes of the painted sweetlips, Diagramma labiosum (Perciformes: Haemulidae), from the southern Great Barrier Reef, including a new genus and three new species. Syst Parasitol 88:195–211
Sey O (1996) Description of Bianium arabicum sp. n. (Trematoda, Lepocreadiidae) from the pufferfish, Lagocephalus lunaris (Bloch & Schneider, 1801) in Kuwait and a review of the genus Bianium Stunkard, 1930. Parasit Hung 28:13–20
Snyder SD, Tkach VV (2001) Phylogenetic and biogeographical relationships among some Holarctic frog lung flukes (Digenea: Haematoloechidae). J Parasitol 87:1433–1440
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stossich M (1890) Brani de elmintologia tergestina, Ser. VII. Boll Soc Adr Sc Nat Trieste 12:39–46
Tkach VV, Pawlowski J, Mariauxc J, Swiderski Z (2001) Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of Platyhelminthes. Taylor & Francis, London, pp 186–193
Wang P-Q (1982) Some digenetic trematodes of marine fishes from Fujian Province, China. Oceanol Limnol Sin 13:179–194
Wee NQ-X, Cribb TH, Bray RA, Cutmore SC (2017a) Two known and one new species of Proctoeces from Australian teleosts: variable host-specificity for closely related species identified through multi-locus molecular data. Parasitol Int 66:16–26
Wee NQ-X, Cutmore SC, Yong RQ-Y, Cribb TH (2017b) Two new and one known species of Tergestia Stossich, 1899 (Trematoda: Fellodistomidae) with novel molecular characterisation for the genus. Syst Parasitol 94:861–874
Wee NQ-X, Cutmore SC, Cribb TH (2018) Two monorchiid species from the freckled goatfish, Upeneus tragula (Perciformes: Mullidae), in Moreton Bay, Australia, including a proposal of a new genus. Syst Parasitol 95:353–365
Wee NQ-X, Cutmore SC, Cribb TH (2019) Four new monorchiids from the golden trevally, Gnathanodon speciosus (Forsskål) (Perciformes: Carangidae), in Moreton Bay, Australia. Syst Parasitol 96:265–278
Yamaguti S (1934) Studies on the helminth fauna of Japan. Part II. Trematodes of fishes, I. Jpn J Zoo 5:249–541
Yamaguti S (1938) Studies on the helminth fauna of Japan. Part 21. Trematodes of fishes, IV. Satyu Yamaguti, Kyoto
Yamaguti S (1953) Systema Helminthum. Part I Digenetic Trematodes of Fishes. AIBS Bulletin 4(3).
Zhukov EV (1983) New representatives of the fauna of trematodes from the fishes of the Gulf of Mexico. Parazitologiya 17:112–117
Acknowledgements
We thank all members of the Marine Parasitology Laboratory for their assistance with the collection of fish and the staff of Heron Island Research Station and Lizard Island Research Station in Australia and CRIOBE Research Station in French Polynesia for their support of our work.
Funding
This project was supported by The Holsworth Wildlife Research Endowment & The Ecological Society of Australia, The Joyce W. Vickery Research Fund administered by The Linnean Society of New South Wales, and the PADI Foundation through funds awarded to NW. NW is supported by a PhD scholarship from the University of Queensland (Research Training Program (RTP) Scholarship).
Author information
Authors and Affiliations
Contributions
PS collected samples in the field. NW and TC conceived the research. NW conducted morphological and molecular analyses. NW, SC and TC analysed the data. NW wrote the manuscript. All authors read and approved the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Data availability
The datasets generated during and/or analysed during the current study are available in GenBank repository, https://www.ncbi.nlm.nih.gov/nucleotide. GenBank accession numbers are listed in Table 1.
Additional information
Communicated by M. Curini-Galletti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/C6963209-8B5D-4E24-80E0-0C6D8D5EE26D.
Rights and permissions
About this article
Cite this article
Wee, N.QX., Cutmore, S.C., Sasal, P. et al. Three new species of Allobacciger Hafeezullah & Siddiqi, 1970 (Digenea: Monorchiidae) from Australia and French Polynesia. Mar. Biodivers. 50, 16 (2020). https://doi.org/10.1007/s12526-019-01029-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-019-01029-8