Abstract
Purpose
Modeling of hemolysis due to fluid stresses faces significant methodological challenges, particularly in geometries with turbulence or complex flow patterns. It is currently unclear how existing phenomenological blood-damage models based on laminar viscous stresses can be implemented into turbulent computational fluid dynamics simulations. The aim of this work is to generalize the existing laminar models to turbulent flows based on first principles, and validate this generalization with existing experimental data.
Methods
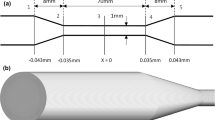
A novel analytical and numerical framework for the simulation of flow-induced hemolysis based on the intermittency-corrected turbulent viscous shear stress (ICTVSS) is introduced. The proposed large-eddy simulation framework is able to seamlessly transition from laminar to turbulent conditions in a single flow domain by linking laminar shear stresses to dissipation of mechanical energy, accounting for intermittency in turbulent dissipation, and relying on existing power-law hemolysis models. Simulations are run to reproduce previously published hemolysis data with bovine blood in a benchmark geometry. Two sets of experimental data are relied upon to tune power-law parameters and justify that tuning. The first presents hemolysis measurements in a simple laminar flow, and the second is hemolysis in turbulent flow through the FDA benchmark nozzle. Validation is performed by simulation of blood injected into a turbulent jet of phosphate-buffered saline, with modifications made to account for the local concentration of blood.
Results
Hemolysis predictions are found to be very sensitive to power-law parameters in the turbulent case, though a set of parameters is presented that both matches the turbulent data and is well-justified by the laminar data. The model is shown to be able to predict the general behavior of hemolysis in a second turbulent case. Results suggest that wall shear may play a dominant role in most cases.
Conclusion
The ICTVSS framework of generalizing laminar power-law models to turbulent flows shows promise, but would benefit from further numerical validation and carefully designed experiments.

















Similar content being viewed by others
References
Antiga, L., and D. A. Steinman. Rethinking turbulence in blood. Biorheology 46(2):77–81, 2009.
Blackshear, P. L., F. D. Dorman, and J. H. Steinbach. Some mechanical effects that inuence hemolysis. ASAIO J. 11(1):104–111, 1965.
Bludszuweit, C. Three-dimensional numerical prediction of stress loading of blood particles in a centrifugal pump. Artif. Organs 19(7):590–596, 1995.
Bluestein, M., and L. F. Mockros. Hemolytic effects of energy dissipation in owing blood. Med. Biol. Eng. 7(1):1–16, 1969.
Burton, G. C. Large-eddy simulation of passive-scalar mixing using multifractal subgridscale modeling. Ann Res Briefs 25:211–222, 2005.
Burton, G. C., and W. J. A. Dahm. Multifractal subgrid-scale modeling for large-eddy simulation. I. Model development and a priori testing. Phys. Fluids 17(7):075111, 2005.
Cerutti, S., and C. Meneveau. Intermittency and relative scaling of subgrid-scale energy dissipation in isotropic turbulence. Phys. Fluids 10(4):928–937, 1998.
Craven, B. A., K. I. Aycock, L. H. Herbertson, and R. A. Malinauskas. A cfd-based kriging surrogate modeling approach for predicting device-specific hemolysis power law coefficients in blood-contacting medical devices. Biomech. Model Mech. 30:1–26, 2019.
Cysyk, J., J. B. Clark, R. Newswanger, C. S. Jhun, J. Izer, H. Finicle, J. Reibson, B. Doxtater, W. Weiss, and G. Rosenberg. Chronic in vivo test of a right heart replacement blood pump for failed fontan circulation. ASAIO J. 65(6):593–600, 2019.
Davidson, P. A. Turbulence. Oxford: Oxford University Press, 2004.
Ding, J., S. Niu, Z. Chen, T. Zhang, B. P. Griffth, and Z. J. Wu. Shear-induced hemolysis: species differences. Artif. Organs 39(9):795–802, 2015.
Faghih, M. M., and M. K. Sharp. Extending the power-law hemolysis model to complex ows. J. Biomed. Eng. 138(12):124504, 2016.
Faghih, M. M., and M. K. Sharp. Characterization of erythrocyte membrane tension for hemolysis prediction in complex ows. Biomech. Model Mech. 17(3):827–842, 2018.
Faghih, M. M., and M. K. Sharp. On eulerian versus lagrangian models of mechanical blood damage and the linearized damage function. Artif. Organs 43(7):681–687, 2019.
Forstrom R. J. A new measure of erythrocyte membrane strength—the jet fragility test. University of Minnesota, Minnesota, 1970
Garon, A., and M. I. Farinas. Fast three-dimensional numerical hemolysis approximation. Artif Organs 28(11):1016–1025, 2004.
Giersiepen, M., L. J. Wurzinger, R. Opitz, and H. Reul. Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int. J. Artif. Organs 13(5):300–306, 1990.
Goubergrits, L., J. Osman, R. Mevert, U. Kertzscher, W. K. Pothkow, and H. C. Hege. Turbulence in blood damage modeling. Int. J. Artif. Organs 39(4):160–165, 2016.
Grigioni, M., C. Daniele, U. Morbiducci, G. D’Avenio, G. Di Benedetto, and V. Barbaro. The power-law mathematical model for blood damage prediction: analytical developments and physical inconsistencies. Artif. Organs 28(5):467–475, 2004.
Grigioni, M., U. Morbiducci, G. D’Avenio, G. Di Benedetto, and C. Del Gaudio. A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech. Model Mech. 4(4):249–260, 2005.
Hariharan, P., M. Giarra, V. Reddy, S. W. Day, K. B. Manning, S. Deutsch, S. F. C. Stewart, M. R. Myers, M. R. Berman, G. W. Burgreen, E. G. Paterson, and R. A. Malinauskas. Multilaboratory particle image velocimetry analysis of the fda benchmark nozzle model to support validation of computational uid dynamics simulations. J. Biomed. Eng. 133(4):041002, 2011.
Herbertson, S. E., L. H. Olia, A. Daly, C. P. Noatch, M. V. Smith, and Malinauskas R. A. Kameneva. Multilaboratory study of ow-induced hemolysis using the fda benchmark nozzle model. Artif. Organs 39(3):237–248, 2015.
Hund, S. J., J. F. Antaki, and M. Massoudi. On the representation of turbulent stresses for computing blood damage. Int. J. Eng. Sci. 48(11):1325–1331, 2010.
Jhun, C. S., M. A. Stauer, J. D. Reibson, E. E. Yeager, R. K. Newswanger, J. O. Taylor, K. B. Manning, W. J. Weiss, and G. Rosenberg. Determination of reynolds shear stress level for hemolysis. ASAIO J. 64(1):63–69, 2018.
Johnson, P. L., and C. Meneveau. Predicting viscous-range velocity gradient dynamics in largeeddy simulations of turbulence. J. Fluid Mech. 837:80–114, 2018.
Jones, S. A. A relationship between reynolds stresses and viscous dissipation: implications to red cell damage. Ann. Biomed. Eng. 23(1):21–28, 1995.
Kaul, C. M., and V. Raman. A posteriori analysis of numerical errors in subfilter scalar variance modeling for large eddy simulation. Phys. Fluids 23(3):035102, 2011.
Kolmogorov, A. N. The local structure of turbulence in incompressible viscous uid for very large reynolds numbers. Cr. Acad. Sci. URSS 30:301–305, 1941.
Lesieur, M., and O. Metais. New trends in largeeddy simulations of turbulence. Ann. Rev. Fluid Mech. 28(1):45–82, 1996.
Marom, G., and D. Bluestein. Lagrangian methods for blood damage estimation in cardiovascular devices-how numerical implementation affects the results. Expert Rev. Med. Dev. 13(2):113–122, 2016.
Meneveau, C., and J. Katz. Scale-invariance and turbulence models for large-eddy simulation. Ann. Rev. Fluid Mech. 32(1):1–32, 2000.
Meneveau, C., and J. O’Neil. Scaling laws of the dissipation rate of turbulent subgrid-scale kinetic energy. Phys. Rev. E 49(4):2866, 1994.
Morshed, K. N., D. Bark, Jr, M. Forleo, and L. P. Dasi. Theory to predict shear stress on cells in turbulent blood ow. PLoS ONE 9(8):e105357, 2014.
Nelkin, M. Multifractal scaling of velocity derivatives in turbulence. Phys. Rev. A 42(12):7226–7229, 1990.
Nicoud, F., and F. Ducros. Subgrid-scale stress modelling based on the square of the velocity gradient tensor. Flow Turbul. Combust. 62(3):183–200, 1999.
Ozturk, M., E. O’Rear, and D. Papavassiliou. Reynolds stresses and hemolysis in turbulent ow examined by threshold analysis. Fluids 1(4):42, 2016.
Pope, S. B. Turbulent Flows. Cambridge: Cambridge University Press, 2000.
Quinlan, N. J., and P. N. Dooley. Models of owinduced loading on blood cells in laminar and turbulent ow, with application to cardiovascular device ow. Ann. Biomed. Eng. 35(8):1347–1356, 2007.
Ravichandran, A. K., J. Parker, E. Novak, S. M. Joseph, J. D. Schilling, G. A. Ewald, and S. Silvestry. Hemolysis in left ventricular assist device: a retrospective analysis of outcomes. J. Heart Lung Transpl. 33(1):44–50, 2014.
Roache, P. J. Perspective: a method for uniform reporting of grid refinement studies. J. Fluid Eng. 116(3):405–413, 1994.
Sallam, A. M., and N. H. C. Hwang. Human red blood cell hemolysis in a turbulent shear ow: contribution of reynolds shear stresses. Biorheology 21(6):783–797, 1984.
Stewart, S. F., P. Hariharan, E. G. Paterson, G. W. Burgreen, V. Reddy, S. W. Day, M. Giarra, K. B. Manning, S. Deutsch, M. R. Berman, et al. Results of fda’s first interlaboratory computational study of a nozzle with a sudden contraction and conical diffuser. Cardiovasc. Eng. Technol. 4(4):374–391, 2013.
Stewart, S. F. C., E. G. Paterson, G. W. Burgreen, P. Hariharan, M. Giarra, V. Reddy, S. W. Day, K. B. Manning, S. Deutsch, M. R. Berman, M. R. Myers, and R. A. Malinauskas. Assessment of cfd performance in simulations of an idealized medical device: results of fda’s first computational interlaboratory study. Cardiovasc. Eng. Technnol. 3(2):139–160, 2012.
Wu, P., S. GroHardt, F. Boehning, and P. L. Hsu. An energy-dissipation-based power-law formulation for estimating hemolysis. Biomech. Model Mech. 30:1–12, 2019.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under Award Number R01HL136369. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Congressionally Directed Medical Research Programs under Award No. (Grant No. W81XWH-16-1-0536).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Amy L. Throckmorton oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tobin, N., Manning, K.B. Large-Eddy Simulations of Flow in the FDA Benchmark Nozzle Geometry to Predict Hemolysis. Cardiovasc Eng Tech 11, 254–267 (2020). https://doi.org/10.1007/s13239-020-00461-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00461-3