Abstract
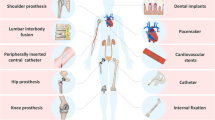
Due to the global aging at an accelerated rate, demands for medical implants have been rapidly increasing. Consequently, a number of complications associated with medical implants has been reported. The complication is mainly due to foreign body response (FBR) which is a nonspecific immune response. Once the implants inserted inside the body, the immune system recognize the implant as foreign body and the immune response cause several problems that could possibly lead to mortality. Conventional approaches including medication have limitations in terms of effectiveness, thus various strategies to reduce the FBR have been studied. Herein, we review current trends of research to reduce FBR, and discuss how the strategies work and function in the body. The strategies include usage of biomaterials that have similar degree of softness to the body, effective drug delivery techniques, and biomimetic surfaces. Furthermore, the possible applications and future perspectives on the strategies will be provided.




Similar content being viewed by others
References
Onuki, Y., Bhardwaj, U., Papadimitrakopoulos, F. & Burgess, D.J. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J. Diabetes Sci. Technol.2, 1003–1015 (2008).
Patel, S.R. & Lieber, C.M. Precision electronic medicine in the brain. Nat. Biotechnol.37, 1007–1012 (2019).
Wilson, G.S., Zhang, Y., Reach, G., Moatti-Sirat, D., Poitout, V., Thévenot, D.R., Lemonnier, F. & Klein, J.-C. Progress toward the development of an implantable sensor for glucose. Clin. Chem.38, 1613–1617 (1992).
do Carmo Da Costa, S.S., Neto, A.S., Costa, R., Caldas, J.G. & Filho, M.M. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: A 6-month follow-up prospective study. Pacing Clin Electrophysiol.25, 1301–1306 (2002).
Mulpuru, S.K., Madhavan, M., McLeod, C.J., Cha, Y.-M. & Friedman, P.A. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J. Am. Coll. Cardiol.69, 189–210 (2017).
Belverud, S., Mogilner, A. & Schulder M. Intrathecal pumps. Neurotherapeutics5, 114–122 (2008).
Frazier, O. & Jacob, L.P. Small pumps for ventricular assistance: progress in mechanical circulatory support. Cardiol. Clin.25, 553–564 (2007).
Katti, K.S. Biomaterials in total joint replacement. Colloids Surf., B39, 133–142 (2004).
Long M, Rack H. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials19, 1621–1639 (1998).
Lee, H.J., Son, Y., Kim, J., Lee, C.J., Yoon, E.-S. & Cho, I.-J. A multichannel neural probe with embedded microfluidic channels for simultaneous in vivo neural recording and drug delivery. Lab Chip15, 1590–1597 (2015).
Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regener. Biomater.3, 107–110 (2016).
Morais, J.M., Papadimitrakopoulos, F. & Burgess, D.J. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J.12, 188–196 (2010).
Anderson, J.M., Rodriguez, A. & Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol.20, 86–100 (2008).
Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res.31, 81–110 (2001).
Fournier, E., Passirani, C., Montero-Menei, C. & Benoit, J. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials24, 3311–3331 (2003).
Cotran, R.S. & Pober, J.S. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J. Am. Soc. Nephrol.1, 225–235 (1990).
Jutila M.A. Leukocyte traffic to sites of inflammation. APMIS100, 191–201 (1992).
Pober, J.S. & Cotran, R.S. The role of endothelial cells in inflammation. Transplantation50, 537–544 (1990).
Johnston, Jr., R.B. Monocytes and macrophages. N. Engl. J. Med.318, 747–752 (1988).
Williams, G. & Williams, W.J. Granulomatous inflammation—a review. J. Clin. Pathol.36, 723–733 (1983).
Thompson, J.A., Anderson, K.D., Di Pietro, J.M., Zwiebel, J.A., Zametta, M., Anderson, W.F. & Maciag, T. Site-directed neovessel formation in vivo. Science241, 1349–1352 (1988).
Rhodes, N., Hunt, J. & Williams, D. Macrophage subpopulation differentiation by stimulation with biomaterials. J. Biomed. Mater. Res.37, 481–488 (1997).
Luster, A.D. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med.338, 436–445 (1998).
Mc. Nally, A.K., Jones, J.A., Mac. Ewan, S.R., Colton, E. & Anderson, J.M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res., Part A86, 535–543 (2008).
Sullivan, D.E., Ferris, M., Nguyen, H., Abboud, E. & Brody, A.R. TNF-α induces TGF-β1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J. Cell. Mol. Med.13, 1866–1876 (2009).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest.122, 787–795 (2012).
Luttikhuizen, D.T., Harmsen, M.C. & Luyn, M.J.V. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng.12, 1955–1970 (2006).
Anderson, J.M. & Langone, J.J. Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J. Controlled Release57, 107–113 (1999).
Tijero, M., Gabriel, G., Caro, J., Altuna, A., Hernández, R., Villa, R., Berganzo, J., Blanco, F., Salido, R. & Fernández, L. SU-8 microprobe with microelectrodes for monitoring electrical impedance in living tissues. Biosens. Bioelectron.24, 2410–2416 (2009).
Cheung, K.C., Renaud, P., Tanila, H. & Djupsund, K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens. Bioelectron.22, 1783–1790 (2007).
Mercanzini, A., Cheung, K., Buhl, D.L., Boers, M., Maillard, A., Colin, P., Bensadoun, J.-C., Bertsch, A. & Renaud, P. Demonstration of cortical recording using novel flexible polymer neural probes. Sens. Actuators, A143, 90–96 (2008).
Takeuchi, S., Ziegler, D., Yoshida, Y., Mabuchi, K. & Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip5, 519–523 (2005).
Altuna, A., Bellistri, E., Cid, E., Aivar, P., Gal, B., Berganzo, J., Gabriel, G., Guimerà, A., Villa, R. & Fernández, L.J. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab Chip13, 1422–1430 (2013).
Lee, S.M., Byeon, H.J., Kim, B.H., Lee, J., Jeong, J.Y., Lee, J.H., Moon, J.-H., Park, C., Choi, H. & Lee S.-H. Flexible and implantable capacitive microelectrode for bio-potential acquisition. BioChip J.11, 153–163 (2017).
Trada, H.V., Vendra, V., Tinney, J.P., Yuan, F., Jackson, D.J., Walsh, K.M. & Keller, B.B. Implantable thin-film porous microelectrode array (P-MEA) for electrical stimulation of engineered cardiac tissues. BioChip J.l9, 85–94 (2015).
Huang, S.-H., Lin, S.-P. & Chen, J.-J.J. In vitro and in vivo characterization of SU-8 flexible neuroprobe: From mechanical properties to electrophysiological recording. Sens. Actuators, A216, 257–265 (2014).
Lee, K.-K., He, J., Singh, A., Massia, S., Ehteshami, G., Kim, B. & Raupp, G. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng.14, 32 (2003).
Chen, Y.-Y., Lai, H.-Y., Lin, S.-H., Cho, C.-W., Chao, W.-H., Liao, C.-H., Tsang, S., Chen, Y.-F. & Lin, S.-Y. Design and fabrication of a polyimide-based microelectrode array: application in neural recording and repeatable electrolytic lesion in rat brain. J. Neurosci. Methods182, 6–16 (2009).
Suzuki, T., Mabuchi, K. & Takeuchi, S. A 3D flexible parylene probe array for multichannel neural recording. Proc of the 1rst International IEEE EMBS Conf on Neural Eng. DOI https://doi.org/10.1109/CNE.2003.1196780
Wu, F., Im, M. & Yoon, E. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, TRANSDUCERS’11. DOI https://doi.org/10.1109/TRANSDUCERS.2011.5969356 (2011).
Gilgunn, P., Khilwani, R., Kozai, T., Weber, D., Cui, X., Erdos, G., Ozdoganlar, O. & Fedder, G. An ultracompliant, scalable neural probe with molded biodissolvable delivery vehicle. Proceedings of the IEEE international conference on Micro Electro Mechanical Systems (MEMS). DOI https://doi.org/10.1109/MEMSYS.2012.6170092 (2012).
Jeon, M., Cho, J., Kim, Y.K., Jung, D., Yoon, E.-S., Shin, S. & Cho, I.-J. Partially flexible MEMS neural probe composed of polyimide and sucrose gel for reducing brain damage during and after implantation. J. Micromech. Microeng.24, 025010 (2014).
de Vos, P., Hoogmoed, C.G. & Busscher, H.J. Chemistry and biocompatibility of alginate-PLL capsules for immunoprotection of mammalian cells. J. Biomed. Mater. Res.60, 252–259 (2002).
Khor, E. & Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials24, 2339–2349 (2003).
de Vos, P., de Haan, B., Wolters, G., Strubbe, J. & van Schilfgaarde, R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia40, 262–270 (1997).
Lee, Y.M., Kim, S. & Kim, S. Synthesis and properties of poly (ethylene glycol) macromer/β-chitosan hydrogels. J. Mater. Sci.: Mater. Med.8, 537–541 (1997).
Gupta, K. & Kumar, M.N.R. pH dependent hydrolysis and drug release behavior of chitosan/poly (ethylene glycol) polymer network microspheres. J. Mater. Sci.: Mater. Med.12, 753–759 (2001).
Turner, R.F., Harrison, D.J. & Rojotte, R.V. Preliminary in vivo biocompatibility studies on perfluorosulphonic acid polymer membranes for biosensor applications. Biomaterials12, 361–368 (1991).
Zhang, Y. & Wilson, G.S. In vitro and in vivo evaluation of oxygen effects on a glucose oxidase based implantable glucose sensor. Anal. Chim. Acta281, 513–520 (1993).
Hetrick, E.M. & Schoenfisch, M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev.35, 780–789 (2006).
Ward, W.K., Slobodzian, E.P., Tiekotter, K.L. & Wood, M.D. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials23, 4185–4192 (2002).
Quinn, C.P., Pathak, C.P., Heller, A. & Hubbell, J.A. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly (ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials16, 389–396 (1995).
Abraham, A.A., Means, A.K., Clubb, Jr., F.J., Fei, R., Locke, A.K., Gacasan, E.G., Coté, G.L. & Grunlan, M.A. Foreign body reaction to a subcutaneously implanted self-cleaning, thermoresponsive hydrogel membrane for glucose biosensors. ACS Biomater. Sci. Eng.4, 4104–4111 (2018).
Dolwick, M.F. & Aufdemorte, T.B. Silicone-induced foreign body reaction and lymphadenopathy after temporomandibular joint arthroplasty. Oral Surg., Oral Med., Oral Pathol.59, 449–452 (1985).
Shaik IH, Gandrapu B, Gonzalez-Ibarra F, Flores D, Matta J, Syed AK. Silicone breast implants: a rare cause of pleural effusion. Case Rep. Pulmonol.2015, 652918 (2015).
Lee, J.S., Shin, B.H., Yoo, B.Y., Nam, S.-Y., Lee, M., Park, H., Choy, Y.B., Heo, C.Y. & Koh, W.-G. Modulation of Foreign Body Reaction Against PDMS Implant by Grafting Topographically Different Poly (Acrylic Acid) Micropatterns. Macromol. Biosci.19, 1900206 (2019).
Kastellorizios, M., Tipnis, N. & Burgess, D.J. Foreign body reaction to subcutaneous implants. Immune Responses to Biosurfaces pp.93–108 Springer (2015).
Cain, D.W. & Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol.17, 233–247 (2017).
Abraham, S.M., Lawrence, T., Kleiman, A., Warden, P., Medghalchi, M., Tuckermann, J., Saklatvala, J. & Clark, A.R. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med.203, 1883–1889 (2006).
Bhardwaj, U., Papadimitrakopoulos, F. & Burgess, D.J. A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors. J. Diabetes Sci. Technol.2, 1016–1029 (2008).
Gancedo, M., Ruiz-Corro, L., Salazar-Montes, A., Rincón, A.R. & Armendáriz-Borunda, J. Pirfenidone prevents capsular contracture after mammary implantation. Aesthetic Plastic Surgery32, 32–40 (2008).
Rujitanaroj, P.-o., Jao, B., Yang, J., Wang, F., Anderson, J.M., Wang, J. & Chew, S.Y. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing. Acta Biomater.9, 4513–4524 (2013).
Takahashi, H., Wang, Y. & Grainger, D.W. Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation. J. Controlled Release147, 400–407 (2010).
Basit, A.W. Advances in colonic drug delivery. Drugs65, 1991–2007 (2005).
Malachowski, K., Breger, J., Kwag, H.R., Wang, M.O., Fisher, J.P., Selaru, F.M. & Gracias, D.H. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew. Chem. Int. Ed.53, 8045–8049 (2014).
Zhang, L., Wang, Y., Yang, Y., Liu, Y., Ruan, S., Zhang Q, Tai, X., Chen, J., Xia, T. & Qiu, Y. High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in breast cancer. ACS Appl. Mater. Interfaces7, 9691–9701 (2015).
Hu, C.-M.J., Fang, R.H., Luk, B.T. & Zhang, L. Polymeric nanotherapeutics: clinical development and advances in stealth functionalization strategies. Nanoscale6, 65–75 (2014).
Vicent, M.J. & Duncan, R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol.24, 39–47 (2006).
Wu, Y., Zhou, D., Zhang, Q., Xie, Z., Chen, X., Jing, X. & Huang, Y. Dual-sensitive charge-conversional polymeric prodrug for efficient codelivery of demethylcantharidin and doxorubicin. Biomacromolecules17, 2650–2661 (2016).
Soni, K.S., Desale, S.S. & Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Controlled Release240, 109–126 (2016).
Oh, J.K., Drumright, R., Siegwart, D.J. & Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci.33, 448–477 (2008).
Pan, Y.-J., Li, D., Jin, S., Wei, C., Wu, K.-Y., Guo, J. & Wang, C.-C. Folate-conjugated poly (N-(2-hydroxypropyl) methacrylamide-co-methacrylic acid) nanohydrogels with pH/redox dual-stimuli response for controlled drug release. Polym. Chem.4, 3545–3553 (2013).
Chiang, Y.-T. & Lo, C.-L. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials35, 5414–5424 (2014).
Santini, Jr., J.T., Cima, M.J. & Langer, R. A controlled-release microchip. Nature397, 335 (1999).
Gensler, H., Sheybani, R., Li, P.-Y., Mann, R.L. & Meng, E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices14, 483–496 (2012).
Farra, R., Sheppard, N.F., McCabe, L., Neer, R.M., Anderson, J.M., Santini, J.T., Cima, M.J. & Langer, R. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl. Med.4, 122 ra121 (2012).
Lee, S.H., Lee, Y.B., Kim, B.H., Lee, C., Cho, Y.M., Kim, S.-N., Park, C.G., Cho, Y.-C. & Choy, Y.B. Implantable batteryless device for on-demand and pulsatile insulin administration. Nat. Commun.8, 15032 (2017).
Barthlott, W. & Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta202, 1–8 (1997).
Feng, L., Li, S., Li, Y., Li, H., Zhang, L., Zhai, J., Song, Y., Liu, B., Jiang, L. & Zhu, D. Super-hydrophobic surfaces: from natural to artificial. Adv. Mater.14, 1857–1860 (2002).
Kim, H., Han, H., Lee, S., Woo, J., Seo, J. & Lee, T. Nonfluorinated Superomniphobic Surfaces through Shape-Tunable Mushroom-like Polymeric Micropillar Arrays. ACS Appl. Mater. Interfaces11, 5484–5491 (2018).
Shin, S., Seo, J., Han, H., Kang, S., Kim, H. & Lee, T. Bio-inspired extreme wetting surfaces for biomedical applications. Materials9, 116 (2016).
Tuteja, A., Choi, W., Ma, M., Mabry, J.M., Mazzella, S.A., Rutledge, G.C., McKinley, G.H. & Cohen, R.E. Designing superoleophobic surfaces. Science318, 1618–1622 (2007).
Tuteja, A., Choi, W., Mabry, J.M., McKinley, G.H. & Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. U. S. A.105, 18200–18205 (2008).
Seo, J., Lee, S.-K., Lee, J., Lee, J.S., Kwon, H., Cho, S.-W., Ahn, J.-H. & Lee, T. Path-programmable water droplet manipulations on an adhesion controlled superhydrophobic surface. Sci. Rep.5, 12326 (2015).
Seo, J., Lee, S., Lee, J. & Lee, T. Guided transport of water droplets on superhydrophobic—hydrophilic patterned Si nanowires. ACS Appl. Mater. Interfaces3, 4722–4729 (2011).
Seo, J., Lee, S., Han, H., Jung, H.B., Hong, J., Song, G., Cho, S.M., Park, C., Lee, W. & Lee, T. Gasdriven ultrafast reversible switching of super-hydrophobic adhesion on palladium-coated silicon nanowires. Adv. Mater.25, 4139–4144 (2013).
Han, H., Lee, J.S., Kim, H., Shin, S., Lee, J., Kim, J., Hou X, Cho, S.-W., Seo, J. & Lee, T. Single-droplet multiplex bioassay on a robust and stretchable extreme wetting substrate through vacuum-based droplet manipulation. ACS nano12, 932–941 (2018).
Nhung Nguyen, T.P., Brunet, P., Coffinier, Y. & Boukherroub, R. Quantitative testing of robustness on superomniphobic surfaces by drop impact. Langmuir26, 18369–18373 (2010).
Shafrin, E.G. & Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers1. J. Phys. Chem.64, 519–524 (1960).
Bocquet, L. & Lauga, E. A smooth future? Nat. Mater.10, 334 (2011).
Scholz, I., Bückins, M., Dolge, L., Erlinghagen, T., Weth, A., Hischen, F., Mayer, J., Hoffmann, S., Riederer, M. & Riedel, M. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J. Exp. Biol.213, 1115–1125 (2010).
Wong, T.-S., Kang, S.H., Tang, S.K., Smythe, E.J., Hatton, B.D., Grinthal, A. & Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature477, 443 (2011).
Cronin, R.E. & Reilly, R.F. Unfractionated heparin for hemodialysis: still the best option. Seminars in dialysis23, 510–515 (2010).
Peppas, N.A. & Langer, R. New challenges in biomaterials. Science263, 1715–1720 (1994).
HND AD, Ashraf S. Low heparinization with heparin-bonded bypass circuits: is it a safe strategy? Ann. Thorac. Surg.63, 663–668 (1997).
Lobato, R.L., Despotis, G.J., Levy, J.H., Shore-Lesserson, L.J., Carlson, M.O. & Bennett-Guerrero, E. Anticoagulation management during cardiopulmonary bypass: a survey of 54 North American institutions. J. Thorac. Cardiovasc. Surg.139, 1665–1666 (2010).
Leslie, D.C., Waterhouse, A., Berthet, J.B., Valentin, T.M., Watters, A.L., Jain, A., Kim, P., Hatton, B.D., Nedder, A. & Donovan, K. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol.32, 1134–1140 (2014).
Lee, J.H., Go, A.K., Oh, S.H., Lee, K.E. & Yuk, S.H. Tissue anti-adhesion potential of ibuprofen-loaded PLLA-PEG diblock copolymer films. Biomaterials26, 671–678 (2005).
Hensman, C., Baty, D., Willis, R. & Cuschieri, A. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg. Endosc.12, 1017–1019 (1998).
Rondinone, J., Brassell, J., Miller III, S.A., Thorne, J.O., Rondinone, D.M., Safabash, J. & Vega F. New electrosurgical ball electrode with nonstick properties. Proc. SPIE 3249, Surgical Applications of Energy. DOI https://doi.org/10.1117/12.304338 (1998).
Kang, S.K., Kim, P.Y., Koo, I.G., Kim, H.Y., Jung, J.C., Choi, M.Y., Lee, J.K. & Collins, G.J. Nonstick polymer coatings for energy-based surgical devices employed in vessel sealing. Plasma Processes Polym.9, 446–452 (2012).
Ceviker, N., Keskil, S. & Baykaner, K. A new coated bipolar coagulator. Acta Neurochir.140, 619–620 (1998).
Mikami, T., Minamida, Y., Koyanagi, I. & Houkin, K. Novel bipolar forceps with protein repellence using gold—polytetrafluoroethylene composite film. Operative Neurosurgery60, ONS–157–ONS–161 (2007).
Zhang, P., Chen, H., Zhang, L. & Zhang, D. Anti-adhesion effects of liquid-infused textured surfaces on high-temperature stainless steel for soft tissue. Appl. Surf. Sci.385, 249–256 (2016).
Acknowledgements
This work was also supported (in part) by the Yonsei University Future-leading Research Initiative of 2019 (RMS2 2019-22-0014) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1C1006720).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lee, D., Park, K. & Seo, J. Recent Advances in Anti-inflammatory Strategies for Implantable Biosensors and Medical Implants. BioChip J 14, 48–62 (2020). https://doi.org/10.1007/s13206-020-4105-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-020-4105-7