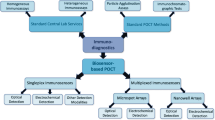
Abstract
Several types of biosensors have been developed to detect a wide variety of human diseases. Immunosensors are classified as the most representative of all biosensors. They are based on antibodies that selectively recognize specific analytes and have high specificity and sensitivity. However, there are limitations to the types of substances that can be detected, and it is sometimes difficult to achieve sufficient sensitivity without additional amplification steps. To overcome these problems, novel immunosensors are being developed that combine DNA-based high signal amplification systems. These technologies ameliorate the low sensitivity of existing immunosensors by using DNA probes that can bind directly to targets as bioreceptors or act as signal amplifiers. In this review, we will discuss immunodetection methods that use DNA-based technologies on laboratory-scale and advanced point-of-care testing (POCT) that employ these technologies for high performance analyses.







Similar content being viewed by others
References
Vigneshvar, S., Sudhakumari, C. C., Senthilkumaran, B. & Prakash, H. Recent advances in biosensor technology for potential applications — an overview. Front. Bioeng. Biotechnol.4, 1–9 (2016).
Turner, A. P. F. Biosensors: Sense and sensibility. Chem. Soc. Rev.42, 3184–3196 (2013).
Kim, T. Y., Lim, M. C., Woo, M. A. & Jun, B. H. Radial flow assay using gold nanoparticles and rolling circle amplification to detect mercuric ions. Nanomaterials8, 1–13 (2018).
Lee, S.H., Park, S.M., Kim, B.N., Kwon, O.S., Rho, W.Y. & Jun, B.H. Emerging ultrafast nucleic acid amplification technologies for next-generation molecular diagnostics. Biosens. Bioelectron.141, 111448 (2019).
Chen, Y., Lu, S., Zhang, S., Li, Y., Qu, Z., Chen, Y., Lu, B., Wang, X. & Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv.3, 1–8 (2017).
Kim, J., Campbell, A.S., de Ávila, B.E.F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol.37, 389–406 (2019).
Bhalla, N., Jolly, P., Formisano, N. & Estrela, P. Introduction to biosensors. Essays Biochem.60, 1–8 (2016).
Ermolaeva, T., Farafonova, O. & Karaseva, N. Possibilities and prospects of immunosensors for a highly sensitive pesticide detection in vegetables and fruits: A review. Food Anal. Methods12, 2785–2801 (2019).
Nguyen, H.H., Lee, S.H., Lee, U.J., Fermin, C.D. & Kim, M. Immobilized enzymes in biosensor applications. Materials (Basel).12, 1–34 (2019).
Liu, Y., Wang, H., Chen, J., Liu, C., Li, W., Kong, J., Yang, P. & Liu, B. A sensitive microchip-based immunosensor for electrochemical detection of low-level biomarker s100b. Electroanalysis25, 1050–1055 (2013).
Juzgado, A., Soldà, A., Ostric, A., Criado, A., Valenti, G., Rapino, S., Conti, G., Fracasso, G., Paolucci, F. & Prato, M. Highly sensitive electrochemiluminescence detection of a prostate cancer biomarker. J. Mater. Chem. B5, 6681–6687 (2017).
Song, C.K., Oh, E., Kang, M.S., Shin, B.S., Han, S.Y., Jung, M., Lee, E.S., Yoon, S.Y., Sung, M.M., Ng, W.B., Cho, N.J. & Lee, H. Fluorescence-based immunosensor using three-dimensional CNT network structure for sensitive and reproducible detection of oral squamous cell carcinoma biomarker. Anal. Chim. Acta1027, 101–108 (2018).
Choi, D.H., Lee, S.K., Oh, Y.K., Bae, B.W., Lee, S.D., Kim, S., Shin, Y.B. & Kim, M.G. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens. Bioelectron.25, 1999–2002 (2010).
Han, G.R. & Kim, M.G. Design, synthesis, and evaluation of gold nanoparticle-antibody-horseradish peroxidase conjugates for highly sensitive chemiluminescence immunoassay (hs-CLIA). Biotechnol. Bioprocess Eng.24, 206–214 (2019).
Bi, S., Zhou, H. & Zhang, S. Multilayers enzymecoated carbon nanotubes as biolabel for ultrasensitive chemiluminescence immunoassay of cancer biomarker. Biosens. Bioelectron.24, 2961–2966 (2009).
Hong, S.Y., Park, Y.M., Jang, Y.H., Min, B.H. & Yoon, H.C. Quantitative lateral-flow immunoassay for the assessment of the cartilage oligomeric matrix protein as a marker of osteoarthritis. Biochip J.6, 213–220 (2012).
Oh, H.K., Kim, J.W., Kim, J.M. & Kim, M.G. High sensitive and broad-range detection of cortisol in human saliva using a trap lateral flow immunoassay (trapLFI) sensor. Analyst143, 3883–3889 (2018).
Ali, J., Najeeb, J., Asim Ali, M., Farhan Aslam, M. & Raza, A. Biosensors: Their fundamentals, designs, types and most recent impactful applications: A review. J. Biosens. Bioelectron.08, 1–9 (2017).
Monošík, R., Stred’anský, M. & Šturdík, E. Biosensors — classification, characterization and new trends. Acta Chim. Slovaca5, 109–120 (2012).
Cosnier, S. Affinity biosensors based on electropolymerized films. Electroanalysis17, 1701–1715 (2005).
Seo, H. Bin & Gu, M.B. Aptamer-based sandwichtype biosensors. J. Biol. Eng.11, 1–7 (2017).
Nezlin, R. Use of aptamers in immunoassays. Mol. Immunol.70, 149–154 (2016).
Lee, S.J., Park, J.W., Kim, I.A., Youn, B.S. & Gu, M.B. Sensitive detection of adipokines for early diagnosis of type 2 diabetes using enzyme-linked antibody-aptamer sandwich (ELAAS) assays. Sens. Actuators, B Chem.168, 243–248 (2012).
Tran, H.V., Piro, B., Reisberg, S., Nguyen, L.H., Nguyen, T.D., Duc, H.T. & Pham, M.C. An electrochemical ELISA-like immunosensor for miRNAs detection based on screen-printed gold electrodes modified with reduced graphene oxide and carbon nanotubes. Biosens. Bioelectron.62, 25–30 (2014).
Darmostuk, M., Rimpelova, S., Gbelcova, H. & Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv.33, 1141–1161 (2014).
Wu, W., Li, J., Pan, D., Li, J., Song, S., Rong, M., Li, Z., Gao, G. & Lu, J. Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of salmonella typhimurium. ACS Appl. Mater. Interfaces6, 16974–16981 (2014).
Santiago-Felipe, S., Tortajada-Genaro, L.A., Puchades, R. & Maquieira, A. Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal. Chim. Acta811, 81–87 (2014).
Zhou, Y., Zhang, H., Liu, L., Li, C., Chang, Z., Zhu, X., Ye, B. & Xu, M. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep.6, 1–8 (2016).
Aswani Kumar, Y.V.V., Renuka, R.M., Achuth, J., Venkataramana, M., Ushakiranmayi, M. & Sudhakar, P. Development of hybrid IgG-aptamer sandwich immunoassay platform for aflatoxin B1 detection and its evaluation onto various field. Front. Pharmacol.9, 271 (2018).
Kang, J., Yeom, G., Ha, S.J. & Kim, M.G. Development of a DNA aptamer selection method based on the heterogeneous sandwich form and its application in a colorimetric assay for influenza A virus detection. New J. Chem.43, 6883–6889 (2019).
Yeom, G., Kang, J., Jang, H., Nam, H.Y., Kim, M.G. & Park, C.J. Development of DNA Aptamers against the nucleocapsid protein of severe fever with thrombocytopenia syndrome virus for diagnostic application: Catalytic signal amplification using replication protein A-conjugated liposomes. Anal. Chem.91, 13772–13779 (2019).
Schweitzer, B., Roverts, S., Grimwade, B., Shao, W., Wang, M., Fu, Q., Shu, Q., Laroche, I., Zhou, Z., Tchernev, V.T., Christiansen, J., Velleca, M. & Kingsmore, S.F. Multiplexed protein profiling on microarrays by rolling-circle amplification. Nat. Biotechnol.20, 359–365 (2002).
He, J., Evers, D.L., O’Leary, T.J. & Mason, J.T. Immunoliposome-PCR: A generic ultrasensitive quantitative antigen detection system. J. Nanobiotechnology10, 1 (2012).
Nussbaum, O., Bar Oz, M., Tilayov, T., Atiya, H. & Dagan, S. A signal amplification probe enhances sensitivity of antibodies and aptamers based Immunodiagnostic assays. J. Immunol. Methods448, 85–90 (2017).
Navrátil, V., Schimer, J., Tykvart, J., Knedlík, T., Vik, V., Majer, P., Konvalinka, J., Šácha, P. DNA-linked Inhibitor Antibody Assay (DIANA) for sensitive and selective enzyme detection and inhibitor screening. Nucleic Acids Res.45, e10 (2017).
Byun, J.Y., Lee, K.H., Shin, Y.B. & Kim, D.M. Cascading amplification of immunoassay signal by cell-free expression of firefly luciferase from detection antibody-conjugated DNA in an escherichia coli extract. ACS Sens.4, 93–99 (2019).
Fischer, C., Wessels, H., Paschke-Kratzin, A. & Fischer, M. Aptamers: Universal capture units for lateral flow applications. Anal. Biochem.522, 53–60 (2017).
Posthuma-Trumpie, G.A., Korf, J. & Van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem.393, 569–582 (2009).
Liu, C.C., Yeung, C.Y., Chen, P.H., Yeh, M.K. & Hou, S.Y. Salmonella detection using 16S ribosomal DNA/RNA probe-gold nanoparticles and lateral flow immunoassay. Food Chem.141, 2526–2532 (2013).
Adhikari, M., Strych, U., Kim, J., Goux, H., Dhamane, S., Poongavanam, M.V., Hagström, A.E.V., Kourentzi, K., Conrad, J.C. & Willson, R.C. Aptamer-phage reporters for ultrasensitive lateral flow assays. Anal. Chem.87, 11660–11665 (2015).
Zhu, X., Kou, F.K., Xu, H., Lin, L., Yang, G. & Lin, Z. A highly sensitive aptamer-immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sens. Actuators, B253, 660–665 (2017).
Kang, J., Yeom, G., Jang, H., Oh, J., Park, C.J. & Kim, M.G. Development of replication protein A-conjugated gold nanoparticles for highly sensitive detection of disease biomarkers. Anal. Chem.91, 10001–10007 (2019).
Frohnmeyer, E., Tuschel, N., Sitz, T., Hermann, C., Dahl, G. T., Schulz, F., Baeumner, A.G. & Fischer, M. Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst144, 1840–1849 (2019).
Phillips, E.A., Moehling, T.J., Bhadra, S., Ellington, A.D. & Linnes, J.C. Strand displacement probes combined with isothermal nucleic acid amplification for instrument-free detection from complex samples. Anal. Chem.90, 6580–6586 (2018).
Fredriksson, S., Gullberg, M., Jarvius, J., Olsson, C., Pietras, K., Gústafsdóttir, S.M., Ostman, A. & Landegren, U. Protein detection using proximity-dependent DNA Ligation Assays. Nat. Biotechnol.20, 473–477 (2002).
Hinman, S.S., McKeating, K.S. & Cheng, Q. DNA Linkers and Diluents for Ultrastable Gold Nanoparticle Bioconjugates in Multiplexed Assay Development. Anal. Chem.89, 4272–4279 (2017).
Roskos, K., Hickerson, A.I., Lu, H.-W., Ferguson, T.M., Shinde, D.N., Klaue, Y. & Niemz, A. Simple system for isothermal DNA amplification coupled to lateral flow detection. PLoS One8, e69355 (2013).
Du, X., Zang, Y., Liu, H., Li, P. & Wang, S. Rapid detection of staphylococcus aureus via recombinase polymerase amplification combined with lateral flow strip. Food Anal. Methods11, 2296–2306 (2018).
Lundberg, M., Thorsen, S.B., Assarsson, E., Villablanca, A., Tran, B., Gee, N., Knowles, M., Nielsen, B.S., Couto, E.G., Martin, R., Nilsson, O., Fermer, C., Schlingemann, J., Christensen, I.J., Nielsen, H.J., Ekström, B., Andersson, C., Gustafsson, M., Brunner, N., Stenvang, J. & Fredriksson, S. Multiplexed homogeneous proximity ligation assays for high-throughput protein biomarker research in serological material. Mol. Cell. Proteomics10, 1–11 (2011).
Kang, Y., Feng, K.J., Chen, J.W., Jiang, J.H., Shen, G.L. & Yu, R.Q. Electrochemical detection of thrombin by sandwich approach using antibody and aptamer. Bioelectrochemistry73, 76–81 (2008).
Guo, L. & Kim, D.H. LSPR biomolecular assay with high sensitivity induced by aptamer-antigen-antibody sandwich complex. Biosens. Bioelectron.31, 567–570 (2012).
Pultar, J., Sauer, U., Domnanich, P. & Preininger, C. Aptamer-antibody on-chip sandwich immunoassay for detection of CRP in spiked serum. Biosens. Bioelectron.24, 1456–1461 (2009).
Huang, Y., Nie, X.M., Gan, S.L., Jiang, J.H., Shen, G.L. & Yu, R. Q. Electrochemical immunosensor of platelet-derived growth factor with aptamer-primed polymerase amplification. Anal. Biochem.382, 16–22 (2008).
Qureshi, A., Gurbuz, Y. & Niazi, J. H. Capacitive aptamer-antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sens. Actuators, B209, 645–651 (2015).
Kim, D., Jetson, R.R. & Krusemark, C.J. A DNA-assisted immunoassay for enzyme activity: Via a DNA-linked, activity-based probe. Chem. Commun.53, 9474–9477 (2017).
Maerle, A.V. Simonova, M.A., Pivovarov, V.D., Voronina, D.V., Drobyazina, P.E., Trofimov, D.Y., Alekseev, L.P., Zavriev, S.K., & Ryazantsev, D.Y. Development of the covalent antibody-DNA conjugates technology for detection of IgE and IgM antibodies by immuno-PCR. PLoS One14, e0209860 (2019).
Chen, R., Sun, Y., Huo, B., Yuan, S., Sun, X., Zhang, M., Yin, N., Fan, L., Yao, W., Wang, J., Han, D., Li, S., Pen, Y., Bai, J., Ning, B., Liang, J., Gao, Z. Highly sensitive detection of ochratoxin A based on bio-barcode immunoassay and catalytic hairpin assembly signal amplification. Talanta208, 120405 (2020).
Shen, G., Zhang, S. & Hu, X. Signal enhancement in a lateral flow immunoassay based on dual gold nanoparticle conjugates. Clin. Biochem.46, 1734–1738 (2013).
Hansen, C.H., Yang, D., Koussa, M.A. & Wong, W.P. Nanoswitch-linked immunosorbent assay (NLISA) for fast, sensitive, and specific protein detection. Proc. Natl. Acad. Sci. U. S. A.114, 10367–10372 (2017).
Acknowledgements
This research was supported by the GIST (Gwangju Institute of Science and Technology), Korea, under the Practical Research and Development support program supervised by the GTI (GIST Technology Institute), Samsung Research Funding & Incubation Center of Samsung Electronics under Project Number SRFC-IT1702-10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kang, J., Kim, MG. Advancements in DNA-assisted Immunosensors. BioChip J 14, 18–31 (2020). https://doi.org/10.1007/s13206-020-4103-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-020-4103-9