Abstract
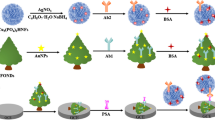
The use of colorimetric bioassays for protein detection is one of the most promising diagnostic approaches, but their relatively poor detection limits have been a critical issue. In this study, we developed an efficient colorimetric bioassay based on antibody-coated peroxidase-mimicking Au nanoparticles (Au NPs) for the detection of prostate-specific antigen (PSA), which is one of the most widely used protein biomarkers for the diagnosis of prostate and breast cancers. Anti-PSA antibody adsorption on the Au NP surface result in 65% of the peroxidasemimicking properties of Au NPs was suppressed to maximize sensitivity of the assay. Anti-PSA antibody adsorption on the Au NP was optimized at 150 µg/mL anti-PSA4 antibody for 1 h incubation, and their surface was blocked with 2% BSA. The experimental conditions of the immunoassay detection were also investigated. A highest assay value was obtained at 10 µg/mL anti-PSA1 capture antibody using 3% BSA for surface blocking and at 2.5 pM anti- PSA4-Ab coated Au NPs for 1 h incubation with PSA antigen. As a result, PSA was detected at a wide range of 0.25 to 2,500 ng/mL with a detection limit of 0.23 ng/mL. The anti-PSA-Ab coated Au NP- based assay was simple, easy to operate, and sensitive. The proposed peroxidase-mimicking anti-PSA Ab-coated Au NP-based assay could be further developed to detect other protein biomarkers.





Similar content being viewed by others
References
Masson, J.-F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. DOI https://doi.org/10.1021/acssensors.6b00763.
Madhavan, B., Yue, S., Galli, U., Rana, S., Gross, W., Müller, M., Giese, N.A., Kalthoff, H., Becker, T., Büchler, M.W. & Zöller, M. Combined evaluation of a panel of protein and miRNA serumexosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer DOI https://doi.org/10.1002/ijc.29324.
Xu, J., Shi, M., Chen, W., Huang, Y., Fang, L., Yao, L., Zhao, S., Chen, Z.-F. & Liang, H. A gold nanoparticle-based four-color proximity immunoassay for one-step, multiplexed detection of protein biomarkers using ribonuclease H signal amplification. Chem. Commun. (Cambridge, U. K.) DOI https://doi.org/10.1039/C7CC09404C.
Wang, Y., Dostalek, J. & Knoll, W. Magnetic nanoparticle-enhanced biosensor based on grating-coupled surface plasmon resonance. Anal. Chem. DOI https://doi.org/10.1021/ac200751s.
Nimse, S.B., Sonawane, M.D., Song, K.-S. & Kim, T. Biomarker detection technologies and future directions. Analyst DOI https://doi.org/10.1039/C5AN01790D.
Geißler, D., Stufler, S., Löhmannsröben, H.-G. & Hildebrandt, N. Six-Color Time-Resolved Förster Resonance Energy Transfer for Ultrasensitive Multiplexed Biosensing. J. Am. Chem. Soc. DOI https://doi.org/10.1021/ja310317n.
Huang, Y., Liu, X., Huang, H., Qin, J., Zhang, L., Zhao, S., Chen, Z.-F. & Liang, H. Attomolar Detection of Proteins via Cascade Strand-Displacement Amplification and Polystyrene Nanoparticle Enhancement in Fluorescence Polarization Aptasensors. Anal. Chem. 87, 8107–8114 (2015). DOI https://doi.org/10.1021/ac5041692.
Akhavan-Tafti, H., Binger, D.G., Blackwood, J.J., Chen, Y., Creager, R.S., de Silva, R., Eickholt, R.A., Gaibor, J.E., Handley, R.S., Kapsner, K.P., Lopac, S. K., Mazelis, M. E., McLernon, T. L., Mendoza, J. D., Odegaard, B. H., Reddy, S. G., Salvati, M., Schoenfelner, B. A., Shapir, N., Shelly, K. R., Todtleben, J. C., Wang, G. & Xie, W. A homogeneous chemiluminescent immunoassay method. J. Am. Chem. Soc. DOI https://doi.org/10.1021/ja312039k.
Krishnan, S., Mani, V., Wasalathanthri, D., Kumar, C.V. & Rusling, J.F. Attomolar detection of a cancer biomarker protein in serum by surface plasmon resonance using superparamagnetic particle labels. Angew. Chem. Int. Ed. DOI https://doi.org/10.1002/anie.201005607.
Munge, B.S., Coffey, A.L., Doucette, J.M., Somba, B.K., Malhotra, R., Patel, V., Gutkind, J.S. & Rusling, J.F. Nanostructured immunosensor for attomolar detection of cancer biomarker interleukin-8 using massively labeled superparamagnetic particles. Angew. Chem. Int. Ed. DOI https://doi.org/10.1002/anie.201102941.
Fang, Y., Li, Y., Zhang, M., Cui, B., Hu, Q. & Wang, L. A novel electrochemical strategy based on porous 3D graphene-starch architecture and silver deposition for ultrasensitive detection of neuron-specific enolase. Analyst DOI https://doi.org/10.1039/C8AN02230E.
Gao, Z., Hou, L., Xu, M. & Tang, D. Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci. Rep. DOI https://doi.org/10.1038/srep03966
Pham, X.-H., Hahm, E., Kim, T.H., Kim, H.-M., Lee, S.H., Lee, Y.-S., Jeong, D.H. & Jun, B.-H. Enzyme-catalyzed Ag growth on Au nanoparticle-assembled structure for highly sensitive colorimetric immunoassay. Sci. Rep. DOI https://doi.org/10.1038/s41598-018-24664-w.
de la Rica, R. & Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. DOI https://doi.org/10.1038/nnano.2012.186
Li, J., Fu, H.-E., Wu, L.-J., Zheng, A.-X., Chen, G.-N. & Yang, H.-H. General colorimetric detection of proteins and small molecules based on cyclic enzymatic signal amplification and hairpin aptamer probe. Anal. Chem. DOI https://doi.org/10.1021/ac3006186.
Xiao, L., Zhu, A., Xu, Q., Chen, Y., Xu, J. & Weng, J. Colorimetric biosensor for detection of cancer biomarker by Au nanoparticle-decorated Bi2Se3 nanosheets. ACS Appl. Mater. Interfaces DOI https://doi.org/10.1021/acsami.6b15750.
Song, Y., Wei, W. & Qu, X. Colorimetric biosensing using smart materials. Adv. Mater. DOI https://doi.org/10.1002/adma.201101853.
Zhang, M., Qing, G., Xiong, C., Cui, R., Pang, D.-W. & Sun, T. Dual-responsive gold nanoparticles for colorimetric recognition and testing of carbohydrates with a dispersion-dominated chromogenic process. Adv. Mater. DOI https://doi.org/10.1002/adma.201203289.
Lee, J.-S., Lytton-Jean, A.K.R., Hurst, S.J. & Mirkin, C.A. Silver nanoparticle-oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett. DOI https://doi.org/10.1021/nl071108g.
Gao, Z., Xu, M., Hou, L., Chen, G. & Tang, D. Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Anal. Chem. DOI https://doi.org/10.1021/ac401433p.
Malashikhina, N., Garai-Ibabe, G. & Pavlov, V. Unconventional application of conventional enzymatic substrate: first fluorogenic immunoassay based on enzymatic formation of quantum dots. Anal. Chem. DOI https://doi.org/10.1021/ac4011342.
Babaian, R.J., Johnston, D.A., Naccarato, W., Ayala, A., Bhadkamkar, V.A. & Fritsche, H.A.H.A. Jr. The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/ml: relation to biopsy strategy. J. Urol. DOI https://doi.org/10.1016/S0022-5347(05)66519-6.
Bok, R.A. & Small, E.J. Bloodborne biomolecular markers in prostate cancer development and progression. Nat. Rev. Cancer DOI https://doi.org/10.1038/nrc951.
Mannello, F. & Gazzanelli, G. Prostate-specific antigen (PSA/hK3): a further player in the field of breast cancer diagnostics? Breast Cancer Res. DOI https://doi.org/10.1186/bcr302.
Mashkoor, F.C., Al-Asadi, J.N. & Al-Naama, L.M. Serum level of prostate-specific antigen (PSA) in women with breast cancer. Cancer Epidemiol. DOI https://doi.org/10.1016/j.canep.2013.06.009.
Nam, J.-M., Thaxton, C.S. & Mirkin, C.A. Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive Detection of Proteins. Science DOI https://doi.org/10.1126/science.1088755.
Thaxton, C.S., Elghanian, R., Thomas, A.D., Stoeva, S.I., Lee, J.-S., Smith, N.D., Schaeffer, A.J., Klocker, H., Horninger, W., Bartsch, G. & Mirkin, C.A. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc. Natl. Acad. Sci. U. S. A. DOI https://doi.org/10.1073/pnas.0904719106.
Chang, H.J., Kang, H.M., Ko, E., Jun, B.H., Lee, H.Y., Lee, Y.S. & Jeang, D.H. PSA Detection with Femtomolar Sensitivity and a Broad Dynamic Range Using SERS Nanoprobes and an Area-Scanning Method. ACS Sens. DOI https://doi.org/10.1021/acssensors.6b00053.
Chao, C.-H., Wu, C.-S., Huang, C.-C., Liang, J.-C., Wang, H.-T., Tang, P.-T., Lin, L.-Y. & Ko, F.-H. A rapid and portable sensor based on protein-modified gold nanoparticle probes and lateral flow assay for naked eye detection of mercury ion. Microelectron. Eng. DOI https://doi.org/10.1016/j.mee.2012.03.015.
de L. M. de Morais, C., Carvalho, J.C., Sant’Anna, C., Eugênio, M., Gasparotto, L.H.S. & Lima, K.M.G. A low-cost microcontrolled photometer with one color recognition sensor for selective detection of Pb2+ using gold nanoparticles. Anal. Methods DOI https://doi.org/10.1039/C5AY01762A.
Kang, J., Zhang, Y., Li, X., Miao, L. & Wu, A. A rapid colorimetric sensor of clenbuterol based on cysteamine-modified gold nanoparticles. ACS Appl. Mater. Interfaces DOI https://doi.org/10.1021/acsami.5b09079.
Rosi, N.L. & Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. DOI https://doi.org/10.1021/cr030067f.
Elghanian, R., Storhoff, J.J., Mucic, R.C., Letsinger, R.L. & Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science DOI https://doi.org/10.1126/science.277.5329.1078.
Marradi, M., Chiodo, F., García, I. & Penadés, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. DOI https://doi.org/10.1039/C2CS35420A.
Zagorovsky, K. & Chan, W.C.W. A plasmonic DNAzyme strategy for point-of-care genetic detection of infectious pathogens. Angew. Chem. Int. Ed. DOI https://doi.org/10.1002/anie.201208715.
Driskell, J.D., Jones, C.A., Tompkins, S.M. & Tripp, R.A. One-step assay for detecting influenza virus using dynamic light scattering and gold nanoparticles. Analyst DOI https://doi.org/10.1039/C1AN15303J.
Lee, C., Gaston, M.A., Weiss, A.A. & Zhang, P. Colorimetric viral detection based on sialic acid stabilized goldnanoparticles. Biosens. Bioelectron. DOI https://doi.org/10.1016/j.bios.2012.10.067.
Liu, Y., Zhang, L., Wei, W., Zhao, H., Zhou, Z., Zhang, Y. & Liu, S. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst DOI https://doi.org/10.1039/C5AN00407A.
Zhang, Y., McKelvie, I.D., Cattrall, R.W. & Kolev, S.D. Colorimetric detection based on localised surface plasmon resonance of gold nanoparticles: Merits, inherent shortcomings and future prospects. Talanta DOI https://doi.org/10.1016/j.talanta.2016.02.015.
Liu, L., Hao, Y., Deng, D. & Xia, N. Nanomaterials-based colorimetric immunoassays. Nanomaterials DOI https://doi.org/10.3390/nano9030316.
Ronkainen, N.J. & Okon, S.L. Nanomaterial-based electrochemical immunosensors for clinically significant biomarkers. Materials (Basel) DOI https://doi.org/10.3390/ma7064669.
Lee, G., Eom, K., Park, J., Yang, J., Haam, S., Huh, Y.-M., Ryu, J.K., Kim, N.H., Yook, J.I., Lee, S.W., Yoon, D.S. & Kwon, T. Real-time quantitative monitoring of specific peptide cleavage by a proteinase for cancer diagnosis. Angew. Chem. Int. Ed. DOI https://doi.org/10.1002/anie.201108830.
Shi, L., De Paoli, V., Rosenzweig, N. & Rosenzweig, Z. Synthesis and application of quantum dots FRET-based protease sensors. J. Am. Chem. Soc. DOI https://doi.org/10.1021/ja063509o.
Chen, G., Xie, Y., Zhang, H., Wang, P., Cheung, H.-Y., Yang, M., Sun, H. A general colorimetric method for detecting protease activity based on peptide-induced gold nanoparticle aggregation. RSC Adv. DOI https://doi.org/10.1039/C3RA46493H.
Lee, S.H. & Jun, B.H. Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. DOI https://doi.org/10.3390/ijms20040865.
Park, S.Y., Lee, S.M., Kim, G.B. & Kim, Y.-P. Gold nanoparticle-based fluorescence quenching via metal coordination for assaying protease activity. Gold Bull. (Berlin, Ger.) DOI https://doi.org/10.1007/s13404-012-0070-9.
Ingram, A., Byers, L., Faulds, K., Moore, B.D. & Graham, D. SERRS-based enzymatic probes for the detection of protease activity. J. Am. Chem. Soc. DOI https://doi.org/10.1021/ja803655h.
Jun, B.H., Kim, G., Jeong, S., Noh, M.S., Pham, X.H., Kang, H., Cho, M.H., Kim, J.H., Lee, Y.S. & Jeong, D.H. Silica core-based Surface-Enhanced Raman Scattering (SERS) tag: advances in multifunctional SERS nanoprobes for bioimaging and targeting of biomarkers. Bull. Korean Chem. Soc. DOI https://doi.org/10.1002/bkcs.10179.
McVey, C., Logan, N., Thanh, N.T.K., Elliott, C. & Cao, C. Unusual switchable peroxidase-mimicking nanozyme for the determination of proteolytic biomarker. Nano Res. DOI https://doi.org/10.1007/s12274-018-2241-3.
Tseng, C.-W., Chang, H.-Y., Chang, J.-Y. & Huang, C.-C. Detection of mercury ions based on mercury-induced switching of enzyme-like activity of platinum/gold nanoparticles. Nanoscale DOI https://doi.org/10.1039/C2NR31716H.
Hvolbæk, B., Janssens, T.V.W., Clausen, B.S., Falsig, H., Christensen, C.H. & Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today DOI https://doi.org/10.1016/S1748-0132(07)70113-5.
Deng, H.-H., Weng, S.-H., Huang, S.-L., Zhang, L.-N., Liu, A.-L., Lin, X.-H. & Chen, W. Colorimetric detection of sulfide based on target-induced shielding against the peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta DOI https://doi.org/10.1016/j.aca.2014.09.023.
Zhao, D., Chen, C., Lu, L., Yang, F. & Yang, X. A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified gold nanoparticles. Sens. Actuators, B DOI https://doi.org/10.1016/j.snb.2015.04.010.
Hizir, M.S., Top, M., Balcioglu, M., Rana, M., Robertson, N.M., Shen, F., Sheng, J. & Yigit, M.V. Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable Peroxidase. Anal. Chem. DOI https://doi.org/10.1021/acs.analchem.5b03926.
Shah, J., Purohit, R., Singh, R., Karakoti, A.S. & Singh, S. ATP-enhanced peroxidase-like activity of gold nanoparticles. J. Colloid Interface Sci. DOI https://doi.org/10.1016/j.jcis.2015.06.015.
Turkevich, J., Stevenson, P.C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. DOI https://doi.org/10.1039/DF9511100055.
Haiss, W., Thanh, N.T.K., Aveyard, J. & Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. DOI https://doi.org/10.1021/ac0702084.
Vignati, G., Chiecchio, A., Osnaghi, B., Giovanelli, L. & Meloncelli, C. Different biological matrices (serum and plasma) utilization in consolidation processes: evaluation of seven Access immunoassays. Clin. Chem. Lab. Med. DOI https://doi.org/10.1515/CCLM.2008.032
Liu, Y., Zhang, Z., Yu, J., Xie, J. & Li, C.M. A concentration-dependent multicolor conversion strategy for ultrasensitive colorimetric immunoassay with the naked eye. Anal. Chim. Acta DOI https://doi.org/10.1016/j.aca.2017.01.034.
Suaifan, G.A.R.Y., Esseghaier, C., Ng, A. & Zourob, M. Ultra-rapid colorimetric assay for protease detection using magnetic nanoparticle-based biosensors. Analyst DOI https://doi.org/10.1039/C3AN36881E.
Fu, G., Sanjay, S.T. & Li, X. Cost-effective and sensitive colorimetric immunosensing using an iron oxide-to-Prussian blue nanoparticle conversion strategy. Analyst DOI https://doi.org/10.1039/C6AN00254D.
Hahn, J., Kim, E., You, Y. & Choi, Y.J. Colorimetric switchable linker-based bioassay for ultrasensitive detection of prostate-specific antigen as a cancer biomarker. Analyst DOI https://doi.org/10.1039/C9AN00552H.
Lai, W., Tang, D., Zhuang, J., Chen, G. & Yang, H. Magnetic bead-based enzyme-chromogenic substrate system for ultrasensitive colorimetric immunoassay accompanying cascade reaction for enzymatic formation of squaric acid-iron(III) chelate. Anal. Chem. DOI https://doi.org/10.1021/ac500738a.
Cao, C., Li, X., Lee, J. & Sim, S.J. Homogenous growth of gold nanocrystals for quantification of PSA protein biomarker. Biosens. Bioelectron. DOI https://doi.org/10.1016/j.bios.2008.07.046.
Acknowledgements
This work was supported by the KU Research Professor Program of Konkuk University and funded by Ministry of Science, ICT and Future Planning (NRF-2016M3A9B6918892), and the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI17C1264).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pham, XH., Hahm, E., Huynh, KH. et al. Sensitive Colorimetric Detection of Prostate Specific Antigen Using a Peroxidase-Mimicking Anti-PSA Antibody Coated Au Nanoparticle. BioChip J 14, 158–168 (2020). https://doi.org/10.1007/s13206-019-4204-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-019-4204-5