Abstract
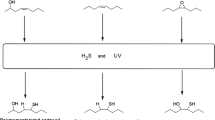
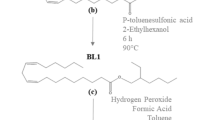
Soy-based fatty acid methyl ester disulfide (FAME-S2) was synthesized in good yield by oxidation of polymercaptanized soybean oil fatty acid methyl ester (PM-FAME). The chemical structure of FAME-S2 is of interest because of its potential as a biobased multi-functional additive in lubricant formulations. Neat FAME-S2 and their blends (1–10% w/w) in polyalphaolefin (PAO-6) and high oleic sunflower oil (HOSuO) were characterized for its chemical, physical and tribological properties. Blends of FAME-S2 in HOSuO relative to similar blends of PM-FAME displayed higher oxidation onset temperature (> 15 °C) that remained constant with increasing concentration. Evaluation of FAME-S2 as an extreme pressure (EP) additive on a 4-ball tribometer showed increasing weld point (WP) with increasing concentration to a maximum of 220 and 180 kgf in HOSuO and PAO-6, respectively, at 10% w/w concentration. The results indicate that FAME-S2 has both anti-oxidant and EP properties and can be applied as a multi-functional biobased additive in lubricant formulation. This work demonstrates an encouraging progress towards the development of effective biobased lubricant additives.










Similar content being viewed by others
References
Swedberg, S., Sullivan, T.: Biobased, biodegradable and food grade lubes. Lubes ‘N’ Greases 25(2), 28–33 (2019)
Sharma, B.K., Biresaw, G. (eds.): Environmentally Friendly and Biobased Lubricants. CRC Press Taylor & Francis Group, Boca Raton (2016)
Biresaw, G., Laszlo, J.A., Evans, K.O., Compton, D.L., Bantchev, G.B.: Synthesis and tribological investigation of lipoyl glycerides. J. Agric. Food Chem. 62, 2233–2243 (2014). https://doi.org/10.1021/jf404289r
Biresaw, G., Compton, D., Evans, K., Bantchev, G.: Lipoate ester multi-functional lubricant additives. Ind. Eng. Chem. Res. 55, 373–383 (2016). https://doi.org/10.1021/acs.iecr.5b03697)
Bantchev, G.B., Moser, B.R., Murray, R.E., Biresaw, G., Hughes, S.R.: Synthesis and characterization of phosphonates from methyl linoleate and vegetable oils. J. Am. Oil Chem. Soc. 93, 1671–1682 (2016)
Biresaw, G., Bantchev, G.B.: Tribological properties of biobased ester phosphonates. J. Am. Oil Chem. Soc. 90, 891–902 (2013)
Biresaw, G., Bantchev, G.: Tribological properties of limonene bisphosphonates. Tribol. Lett. 60(1), 11–25 (2015)
Biresaw, G., Bantchev, G.B., Harry-O’kuru, R.E.: Biobased poly-phosphonate additives from methyl linoleates. Tribol. Trans. 62(3), 428–442 (2019)
Matson, M.S., Refvik, M.D., Netemeyer, E.J., Cameron, C., Marrion, A.R., Wright, A.C.: Methods of mercaptanizing olefinic hydrocarbons and compositions produced therefrom. US Patent 8,461,293 (Assignee: Chevron-Phillips Chemical Company LP The Woodlands, Texas) (2013)
Biresaw, G., Lansing, J., Bantchev, G., Murray, R., Harry-O’kuru, R.: (2017) Tribological Investigation of Polymercaptanized Soybean Oil. Tribol. Lett. 65, 87 (2017). https://doi.org/10.1007/s11249-017-0866-0)
Ionescu, M., Radojcic, D., Wana, X., Petrovic, Z.S., Upshaw, T.A.: Functionalized vegetable oils as precursors for polymers by thiol-ene reaction. Eur. Polym. J. 67, 439–448 (2015)
Javni, I., Bilic, O., Ljubic, D., Wan, X., Petrovic, Z.S., Upshaw, T.A.: Polymercaptan-based polyurethane foams. J. Cell. Plast. 52(6), 643–656 (2016)
Liu, K.T., Tong, Y.C.: A facile conversion of thiols to disulfides. Synthesis 1978, 669–670 (1978)
Evans, B.J., Doi, J.T., Musker, W.K.: 19F NMR Study of the Reaction of p -Fluorobenzenethiol and Disulfide with Periodate and Other Selected Oxidizing Agents. J. Org. Chem. 55, 2337–2344 (1990)
Tam, J.P., Wu, C.-R., Liu, W., Zhang, J.-W.: Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 113, 6657–6662 (1991)
Shi, T., Rabenstein, D.L.: Discovery of a highly selective and efficient reagent for formation of intramolecular disulfide bonds in peptides. J. Am. Chem. Soc. 122(29), 6809–6815 (2000)
Kirihara, M., Asai, Y., Ogawa, S., Noguchi, T., Hatano, A., Hirai, Y.: A mild and environmentally benign oxidation of thiols to disulfides. Synthesis 21, 3286–3289 (2007)
Harutyunyan, R., Rezvani, M.A., Heravi, M.M.: H5[PMo10V2O40] as a green, reusable, and highly efficient catalyst for the oxidation of dithiols in intermolecular reactions using permanganate as an oxidizing reagent. Synth. React. Inorg. Met. Org., Nano-Met. Chem. 41(1), 94–99 (2011)
He, Y., Hang, D., Lu, M.: A simple and practical method for the oxidation of thiols to disulfides at mild conditions without solvents. Phosphorus Sulfur Silicon Relat. Elem. 187(9), 1118–1124 (2012)
Bayraq, S.S., Nikseresht, A., Khosravi, I.: (NH4)6Mo7O24•4H2O as an efficient, selective, and reusable catalyst for the oxidation of thiols to disulfides using potassium bromate. Phosphorus Sulfur Silicon Relat. Elem. 188(9), 1236–1243 (2013)
Lui, Y., Wang, H., Wang, C., Wan, J.-P., Wen, C.: Bio-based green solvent mediated disulfide synthesis via thiol couplings free of catalyst and additive. RSC Adv. 3, 21369–21372 (2013)
Wang, H., Huang, G., Sun, Y., Liu, Y.: Simple conversion of thiols to disulfides in EtOH under ambient aerobic conditions without using any catalyst or additive. J. Chem. Res. 38, 96–97 (2014)
Yuan, J., Ma, X., Yi, H., Liu, C., Lei, A.: I2-catalyzed oxidative C(sp3)–H/S–H coupling: utilizing alkanes and mercaptans as the nucleophiles. Chem. Commun. 50, 14386–14389 (2014)
Shah, S.S., Karthik, S., Singh, N.D.P.: Vis/NIR light driven mild and clean synthesis of disulfides in the presence of Cu2(OH)PO4 under aerobic conditions. RSC Adv. 5(56), 45416–45419 (2015)
Qiu, X., Yang, X., Zhang, Y., Song, S., Jiao, N.: Efficient and practical synthesis of unsymmetrical disulfides via base-catalyzed aerobic oxidative dehydrogenative coupling of thiols. Org. Chem. Front. 6(13), 2220–2225 (2019)
Rossrucker, T., Fessenbecker, A.: Sulfur carriers. In: Rudnick, L.R. (ed.) Lubricant Additives. Chemistry and Applications, pp. 259–291. CRC Press, Boca Raton (2003)
Bantchev, G.B., Biresaw, G., Mohamed, A., Moser, J.: Temperature dependence of the oxidative stability of corn oil and polyalphaolefin in the presence of sulfides. Thermochim. Acta 513(1–2), 94–99 (2011)
Biresaw, G., Asadauskas, S.J., McClure, T.G.: Polysulfide and biobased extreme pressure additive performance in vegetable vs paraffinic base oils. Ind. Eng. Chem. Res. 51(1), 262–273 (2012)
Li, W., Jiang, C., Chao, M., Wang, X.: Natural garlic oil as a high-performance, environmentally friendly, extreme pressure additive in lubricating oils. ACS Sustain. Chem. Eng. 2(4), 798–803 (2014)
Biresaw, G., Lansing, J.C., Bantchev, G.B., Murray, R.E., Harry-O’Kuru, R.E.: Chemical, physical and tribological investigation of polymercaptanized soybean oil. Tribol. Lett. 65(87), 1–16 (2017)
A.O.C.S. Official: Method Te 2a-64, Acid Value, p. 1 (1997)
ASTM D 7042-11a: “Standard test method for dynamic viscosity and density of liquids by Stabinger viscometer (and the Calculation of Kinematic Viscosity),” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 186–193 (2012)
ASTM D 2270-93: “Standard practice for calculating viscosity index from kinematic viscosity at 40 and 100 °C,” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 849–854 (2012)
ASTM D 6186-08: “Standard test method for oxidation induction time of lubricating oils by pressure differential scanning calorimetry (PDSC),” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 52–56 (2012)
ASTM D 97: “Standard test method for pour point of petroleum products,” Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, pp. 100–104 (2012)
ASTM D 2500: “Standard test method for cloud point of petroleum products,” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 949–953 (2012)
ASTM D 2783–88: “Standard test method for measurement of extreme-pressure properties of lubricating fluids (Four-Ball Method),” Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken, pp. 130–137 (2012)
Noor, M.A.M., Sendijarevic, V., Hoong, S.S., Sendijarevic, I., Ismail, T.N.M.T., Hanzah, N.A., Noor, N.M., Palam, K.D.P., Ghazali, R., Hassan, H.A.: Molecular weight determination of palm olein polyols by gel permeation chromatography using polyether polyols calibration. J. Am. Oil Chem. Soc. 93, 721–730 (2016). https://doi.org/10.1007/s11746-016-2812-y
Grubisic, Z., Rempp, P., Benoit, H.: A universal calibration for gel permeation chromatography. Polym. Lett. 5, 753–759 (1967). https://doi.org/10.1002/pol.1967.110050903. Re-published. In: (1996) Journal of Polymer Science, Part B: Polymer Physics, 34:1707-1713. DOI: 10.1002/polb.1996.922
Wyatt, P.J.: Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272, 1–40 (1993)
Braun, J.L., Kadla, J.F.J.: A relatively simple method for calculating Mark-Houwink parameters using basic definitions. Appl. Polym. Sci. 114, 3303–3309 (2009)
Bantchev, G.B., Cermak, S.C., Durham, A.L., Price, N.P.J.: Estolide molecular weight distribution via gel permeation chromatography. J. Am. Oil Chem. Soc. 96, 365–380 (2019)
Mayo, D.W., Miller, F.A., Hannah, R.W.: Course Notes on the interpretation of infrared and Raman Spectra. Wiley, Hoboken (2004)
Silverstein, R.M., Webster, F.X., Kiemle, D.J.: Spectroscopic Identification of Organic Compounds, 7th edn. Wiley, Hoboken (2005)
Biresaw, G., Adharyu, A., Erhan, S.Z., Carriere, C.J.: Friction and adsorption properties of normal and high oleic soybean oils. J. Am. Oil Chem. Soc. 79(1), 53–58 (2002)
Asadauskas, S.J., Biresaw, G., McClure, T.G.: Effects of chlorinated paraffin and ZDDP concentrations on boundary lubrication properties of mineral and soybean oils. Tribol. Lett. 37(2), 111–121 (2010)
Hope, K.: Balancing lubricant properties with vegetable oil and PAO blends. Proceedings USB Lube TAP (2006)
Biresaw, G.: Environmentally friendly lubricant-development programs at USDA. In: Sik, R. (ed.) Environmentally Considerate Lubricants, STP 1575, pp. 1–23. ASTM International, West Conshohocken (2014)
Galiatsatos, V., Neaffer, R.O., Sen, S., Sherman, B.J.: Refractive index, stress-optical coefficient, and optical configuration arameters of polymers. In: Mark, J.E. (ed.) Physical properties of Polymers Handbook, pp. 535–543. AIP Press, Woodbury (1996)
Bala, V., Hartley, R.J., Hughes, L.J.: The influence of chemical structure on the oxidative stability of organic sulfides. Lubr. Eng. 52, 868–873 (1996)
Schey, J.A.: Tribology in Metalworking Friction, Lubrication and Wear. American Society of Metals, Metals Park (1983)
Acknowledgements
The authors are very grateful to Ms. Linda Cao, Ms. Linda Manthey, Mr. Dan Knetzer and Dr. Karl Vermillion for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Biresaw, G., Bantchev, G.B., Lansing, J. et al. Sulfurized Methyl Esters of Soya Fatty Acids: Synthesis and Characterization. Tribol Lett 68, 61 (2020). https://doi.org/10.1007/s11249-020-01292-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-020-01292-y