Abstract
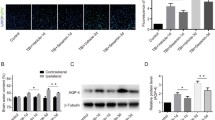
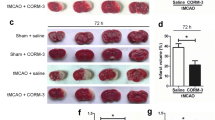
Spinal cord injury (SCI) is a devastating condition of the central nervous system that can lead to permanent motor and sensory deficits. Carbon monoxide–releasing molecule-2 (CORM-2) has been shown to have anti-inflammatory, anti-apoptotic, and angiogenic properties that may be useful for the treatment of SCI. However, it has a short carbon monoxide (CO) release half-life (approximately 1 min). To address this challenge, we developed a CORM-2-incorporated solid lipid nanoparticle (CORM-2-SLN) and evaluated its ameliorating effects for preventing blood-spinal cord barrier (BSCB) disruption and endothelial cell death following SCI. After a moderate compression injury of the spinal cord (compression with a 35-g impounder for 5 min), groups of rats were treated with a CORM-2-solution and CORM-2-SLNs at an equal dose of 10 mg/kg each via an intraperitoneal injection for 8 consecutive days. Behavior analysis was performed and animals were later sacrificed at different time points and evaluated for whether the CORM-2-SLNs prevented BSCB disruption and rescued endothelial cell damage following SCI. The CORM-2-SLN-treated group showed significantly diminished extravasation of Evans Blue dye with enhanced expression of tight junction proteins following SCI. Likewise, significantly diminished endothelial cell markers after SCI were optimally stabilized at 21 days. Additionally, lipopolysaccharide (LPS)-induced loss of tight junction integrity was significantly preserved after CORM-2-SLN treatment in human cerebral microvascular endothelial cell line (hCMEC/D3). Clinically, CORM-2-SLNs were associated with a significantly improved functional recovery, as compared with the CORM-2-solution. CORM-2-SLNs may help potentially to maintain BSCB integrity following SCI.







Similar content being viewed by others
Change history
08 December 2020
A Correction to this paper has been published: <ExternalRef><RefSource>https://doi.org/10.1007/s12035-020-02233-5</RefSource><RefTarget Address="10.1007/s12035-020-02233-5" TargetType="DOI"/></ExternalRef>
References
Ulndreaj A, Badner A, Fehlings MG (2017) Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Research 6
Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57. https://doi.org/10.1016/j.pneurobio.2013.11.002
Wyndaele M, Wyndaele JJ (2006) Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 44(9):523–529. https://doi.org/10.1038/sj.sc.3101893
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 367(9524):1747–1757. https://doi.org/10.1016/S0140-6736(06)68770-9
Kumar H, Jo M-J, Choi H, Muttigi MS, Shon S, Kim B-J, Lee S-H, Han I-B (2018) Matrix Metalloproteinase-8 inhibition prevents disruption of blood–spinal cord barrier and attenuates inflammation in rat model of spinal cord injury. Mol Neurobiol 55(3):2577–2590
Kumar H, Choi H, Jo M-J, Joshi HP, Muttigi M, Bonanomi D, Kim SB, Ban E et al (2018) Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol Commu 6(1):73
Jo M-J, Kumar H, Joshi HP, Choi H, Ko W-K, Kim J, Hwang SS, Park SY et al (2018) Oral administration of α-Asarone promotes functional recovery in rats with spinal cord injury. Front Pharmacol 9:445
Han I-B, Thakor DK, Ropper AE, Yu D, Wang L, Kabatas S, Zeng X, Kim S-W, Zafonte RD, Teng YD (2019) Physical impacts of PLGA scaffolding on hMSCs: Recovery neurobiology insight for implant design to treat spinal cord injury. Experimental neurology:112980
Kraus KH (1996) The pathophysiology of spinal cord injury and its clinical implications. In: Seminars in veterinary medicine and surgery (small animal). vol 4. pp 201–207
Tator CH (1995) Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 5(4):407–413
Abbott NJ (2002) Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat 200(6):629–638
Lee JY, Kim HS, Choi HY, Oh TH, Yune TY (2012) Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood–spinal cord barrier after spinal cord injury. Brain 135(8):2375–2389
Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY (2012) Valproic acid attenuates blood–spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121(5):818–829
Zhou Y, Zheng B, Ye L, Zhang H, Zhu S, Zheng X, Xia Q, He Z et al (2016) Retinoic acid prevents disruption of blood-spinal cord barrier by inducing autophagic flux after spinal cord injury. Neurochem Res 41(4):813–825
Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W (2004) Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24(9):2182–2190
Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ et al (2003) Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma 20(10):1017–1027
Kwon BK, Mann C, Sohn HM, Hilibrand AS, Phillips FM, Wang JC, Fehlings MG (2008) Hypothermia for spinal cord injury. Spine J 8(6):859–874
Sharma HS (2005) Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des 11(11):1353–1389
Shlosberg D, Benifla M, Kaufer D, Friedman A (2010) Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6(7):393–403
Bregman BS, McAtee M, Dai HN, Kuhn PL (1997) Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol 148(2):475–494
Sharma H, Olsson Y, Dey P (1990) Early accumulation of serotonin in rat spinal cord subjected to traumatic injury. Relation to edema and blood flow changes. Neuroscience 36(3):725–730
Mahan VL (2012) Neuroprotective, neurotherapeutic, and neurometabolic effects of carbon monoxide. Medical gas research 2(1):32
Duckers HJ, Boehm M, True AL, Yet S-F, San H, Park JL, Webb RC, Lee M-E et al (2001) Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 7(6):693–698
Foresti R, Clark JE, Green CJ, Motterlini R (1997) Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells involvement of superoxide and peroxynitrite anions. J Biol Chem 272(29):18411–18417
Tschugguel W, Stonek F, Zhegu Z, Dietrich W, Schneeberger C, Stimpfl T, Waldhoer T, Vycudilik W et al (2001) Estrogen increases endothelial carbon monoxide, heme oxygenase 2, and carbon monoxide-derived cGMP by a receptor-mediated system. The Journal of Clinical Endocrinology & Metabolism 86(8):3833–3839
Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI (2001) Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 104(22):2710–2715
Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, Czismadia E, May GA et al (2004) Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J 18(6):771–772
de Rivero Vaccari JP (2019) Carbon monoxide releasing molecule-3 inhibits inflammasome activation: A potential therapy for spinal cord injury. EBioMedicine 40:17–18
Zheng G, Zhan Y, Wang H, Luo Z, Zheng F, Zhou Y, Wu Y, Wang S et al (2019) Carbon monoxide releasing molecule-3 alleviates neuron death after spinal cord injury via inflammasome regulation. EBioMedicine 40:643–654
Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, Rui T (2017) Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol 112(2):16
Joshi HP, Kim SB, Kim S, Kumar H, Jo M-J, Choi H, Kim J, Kyung JW, Sohn S, Kim K-T (2019) Nanocarrier-mediated Delivery of CORM-2 Enhances Anti-allodynic and Anti-hyperalgesic Effects of CORM-2. Molecular neurobiology:1–16
Choi YK, Maki T, Mandeville ET, Koh S-H, Hayakawa K, Arai K, Kim Y-M, Whalen MJ et al (2016) Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med 22(11):1335
Motterlini R, Haas B, Foresti R (2012) Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Med Gas Res 2(1):28. https://doi.org/10.1186/2045-9912-2-28
Adach W, Olas B (2017) The role of CORM-2 as a modulator of oxidative stress and hemostatic parameters of human plasma in vitro. PLoS One 12(9):e0184787. https://doi.org/10.1371/journal.pone.0184787
Motterlini R, Mann BE, Foresti R (2005) Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs 14(11):1305–1318
Qureshi OS, Zeb A, Akram M, Kim M-S, Kang J-H, Kim H-S, Majid A, Han I et al (2016) Enhanced acute anti-inflammatory effects of CORM-2-loaded nanoparticles via sustained carbon monoxide delivery. Eur J Pharm Biopharm 108:187–195
Qureshi OS, Kim H-S, Zeb A, Choi J-S, Kim H-S, Kwon J-E, Kim M-S, Kang J-H et al (2017) Sustained release docetaxel-incorporated lipid nanoparticles with improved pharmacokinetics for oral and parenteral administration. J Microencapsul 34(3):250–261
Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ Res 90(2):e17–e24
Motterlini R, Otterbein LE (2010) The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 9(9):728
Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian G-F et al (2009) Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci 106(30):12489–12493
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201
Jin L, Nation RL, Li J, Nicolazzo JA (2013) Species-dependent blood-brain barrier disruption of lipopolysaccharide: Amelioration by colistin in vitro and in vivo. Antimicrob Agents Chemother 57(9):4336–4342
Chen J-X, Chen Y, DeBusk L, Lin W, Lin PC (2004) Dual functional roles of Tie-2/angiopoietin in TNF-α-mediated angiogenesis. Am J Phys Heart Circ Phys 287(1):H187–H195
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Zhou C, Shi X, Huang H, Zhu Y, Wu Y (2014) Montelukast attenuates neuropathic pain through inhibiting p38 mitogen-activated protein kinase and nuclear factor-kappa B in a rat model of chronic constriction injury. Anesth Analg 118(5):1090–1096
Hervera A, Leánez S, Motterlini R, Pol O (2013) Treatment with carbon monoxide-releasing molecules and an HO-1 inducer enhances the effects and expression of μ-opioid receptors during neuropathic pain. Anesthesiology: The Journal of the American Society of Anesthesiologists 118(5):1180–1197
Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA et al (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6(4):422–428. https://doi.org/10.1038/74680
Zhang X, Shan P, Alam J, Fu XY, Lee PJ (2005) Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem 280(10):8714–8721. https://doi.org/10.1074/jbc.M408092200
Nakao A, Sugimoto R, Billiar TR, McCurry KR (2009) Therapeutic antioxidant medical gas. J Clin Biochem Nutr 44(1):1–13. https://doi.org/10.3164/jcbn.08-193R
Zhang P, Zhang L, Zhu L, Chen F, Zhou S, Tian T, Zhang Y, Jiang X et al (2015) The change tendency of PI3K/Akt pathway after spinal cord injury. Am J Transl Res 7(11):2223
Raghupathi R, Muir JK, Fulp CT, Pittman RN, McIntosh TK (2003) Acute activation of mitogen-activated protein kinases following traumatic brain injury in the rat: Implications for posttraumatic cell death. Exp Neurol 183(2):438–448
Nakahara S, Yone K, Sakou T, Wada S, Nagamine T, Niiyama T, Ichijo H (1999) Induction of apoptosis signal regulating kinase 1 (ASK1) after spinal cord injury in rats: Possible involvement of ASK1-JNK and-p38 pathways in neuronal apoptosis. J Neuropathol Exp Neurol 58(5):442–450
Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE et al (2004) Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. American Journal of Physiology-Renal Physiology 287(5):F979–F989
Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS et al (2003) Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 163(4):1587–1598
Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD (1998) Apoptosis after traumatic human spinal cord injury. J Neurosurg 89(6):911–920
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596. https://doi.org/10.1038/nm.3407
Fan ZK, Lv G, Wang YF, Li G, Yu DS, Wang YS, Zhang YQ, Mei XF et al (2013) The protective effect of salvianolic acid B on blood-spinal cord barrier after compression spinal cord injury in rats. J Mol Neurosci 51(3):986–993. https://doi.org/10.1007/s12031-013-0083-8
Figley SA, Khosravi R, Legasto JM, Tseng YF, Fehlings MG (2014) Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma 31(6):541–552. https://doi.org/10.1089/neu.2013.3034
Whetstone WD, Hsu JYC, Eisenberg M, Werb Z, Noble-Haeusslein LJ (2003) Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J Neurosci Res 74(2):227–239
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M (2011) The blood–spinal cord barrier: Morphology and clinical implications. Ann Neurol 70(2):194–206
Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T et al (2006) A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 12(12):1380–1389. https://doi.org/10.1038/nm1505
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26(50):13007–13016. https://doi.org/10.1523/JNEUROSCI.4323-06.2006
Yoshihara T, Ohta M, Itokazu Y, Matsumoto N, Dezawa M, Suzuki Y, Taguchi A, Watanabe Y et al (2007) Neuroprotective effect of bone marrow-derived mononuclear cells promoting functional recovery from spinal cord injury. J Neurotrauma 24(6):1026–1036. https://doi.org/10.1089/neu.2007.132R
Hansen TM, Moss AJ, Brindle NP (2008) Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res 5(4):236–245
Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C et al (2015) Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348(6232):347–352
Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O (2005) Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 25(19):4694–4705
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology Research & Development Project, Ministry of Health and Welfare, Republic of Korea (HR16C0002), and the National Research Foundation of Korea (NRF-2017R1C1B1011397, NRF-2017R1A2B4006458).
Author information
Authors and Affiliations
Contributions
All authors contributed and critically reviewed and approved the manuscript. IBH and HK conceived and directed the project. IBH, HK, HPJ designed the whole experimental plan. HPJ, UYC, YCL, HC, JK, JWK, and YCK performed the experiment. SS and KK analyzed the data and interpreted the result. JKK formulate and characterized the in vitro CO release properties of CORM-2-SLNs. HPJ, UYC, and IBH prepared the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2157 kb)
Rights and permissions
About this article
Cite this article
Joshi, H.P., Kumar, H., Choi, U.Y. et al. CORM-2-Solid Lipid Nanoparticles Maintain Integrity of Blood-Spinal Cord Barrier After Spinal Cord Injury in Rats. Mol Neurobiol 57, 2671–2689 (2020). https://doi.org/10.1007/s12035-020-01914-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01914-5