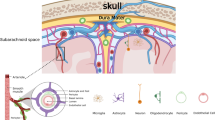
Abstract
Stroke is the leading cause of disability among adults as well as the 2nd leading cause of death globally. Ischemic stroke accounts for about 85% of strokes, and currently, tissue plasminogen activator (tPA), whose therapeutic window is limited to up to 4.5 h for the appropriate population, is the only FDA approved drug in practice and medicine. After a stroke, a cascade of pathophysiological events results in the opening of the blood-brain barrier (BBB) through which further complications, disabilities, and mortality are likely to threaten the patient’s health. Strikingly, tPA administration in eligible patients might cause hemorrhagic transformation and sustained damage to BBB integrity. One must, therefore, delineate upon stroke onset which cellular and molecular factors mediate BBB permeability as well as what key roles BBB rupture plays in the pathophysiology of stroke. In this review article, given our past findings of mechanisms underlying BBB opening in stroke animal models, we elucidate cellular, subcellular, and molecular factors involved in BBB permeability after ischemic stroke. The contribution of each factor to stroke severity and outcome is further discussed. Determinant factors in BBB permeability and stroke include mitochondria, miRNAs, matrix metalloproteinases (MMPs), immune cells, cytokines, chemokines, and adhesion proteins. Once these factors are interrogated and their roles in the pathophysiology of stroke are determined, novel targets for drug discovery and development can be uncovered in addition to novel therapeutic avenues for human stroke management.



Similar content being viewed by others
References
Abbott N (1992) Comparative physiology of the blood-brain barrier. Physiology and pharmacology of the blood-brain barrier. Springer, Berlin, pp 371–396
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7(3):243–253
Afonso-Grunz F, Muller S (2015) Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci 72(16):3127–3141. https://doi.org/10.1007/s00018-015-1922-2
Alghisi GC, Ponsonnet L, Rüegg C (2009) The integrin antagonist cilengitide activates αVβ3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One 4(2):e4449
Allahtavakoli M, Amin F, Esmaeeli-Nadimi A, Shamsizadeh A, Kazemi‐Arababadi M, Kennedy D (2015) Ascorbic acid reduces the adverse effects of delayed administration of tissue plasminogen activator in a rat stroke model. Basic Clin Pharmacol Toxicol 117(5):335–339
Alluri H, Stagg HW, Wilson RL, Clayton RP, Sawant DA, Koneru M, Beeram MR, Davis ML, Tharakan B (2014) Reactive oxygen species-caspase‐3 relationship in mediating blood–brain barrier endothelial cell hyperpermeability following oxygen–glucose deprivation and reoxygenation. Microcirculation 21(2):187–195
Almutairi MM, Gong C, Xu YG, Chang Y, Shi H (2016) Factors controlling permeability of the blood–brain barrier. Cell Mol Life Sci 73(1):57–77
Amantea D, Corasaniti M, Mercuri N, Bernardi G, Bagetta G (2008) Brain regional and cellular localization of gelatinase activity in rat that have undergone transient middle cerebral artery occlusion. Neuroscience 152(1):8–17
Bandopadhyay R, Orte C, Lawrenson J, Reid A, De Silva S, Allt G (2001) Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol 30(1):35–44
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Becker KJ (2002) Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6. 5) in acute stroke. Curr Med Res Opin 18(sup2):s18–s22
Beermann J, Piccoli M-T, Viereck J, Thum T (2016) Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96(4):1297–1325
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood-brain barrier. Peptide transport and delivery into the central nervous system. Springer, Berlin, pp 39–78
Berger C, Fiorelli M, Steiner T, Schäbitz W-Rd, Bozzao L, Bluhmki E, Hacke W von, Kummer Rd (2001) Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke 32(6):1330–1335
Berrier AL, Yamada KM (2007) Cell–matrix adhesion. J Cell Physiol 213(3):565–573
Bihl J, Wang J, Ma X, Yang Y, Zhao B, Chen Y (2018) Exosome and MiRNA in Stroke. In: Cellular and Molecular Approaches to Regeneration and Repair. Springer, Berlin pp 325–361
Borlongan CV, Nguyen H, Lippert T, Russo E, Tuazon J, Xu K, Lee JY, Sanberg PR, Kaneko Y, Napoli E (2019) May the force be with you: Transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab 39(2):367–370. https://doi.org/10.1177/0271678x18811277
Brightman MW, Kadota Y (1993) Nonpermeable and permeable vessels of the brain. NIDA Res Monogr 120:87–87
Brown EJ (1997) Adhesive interactions in the immune system. Trends Cell Biol 7(7):289–295
Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X (2016) MiR-34a regulates blood–brain barrier permeability and mitochondrial function by targeting cytochrome c. J Cereb Blood Flow Metab 36(2):387–392
Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA (2011) Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke 42(5):1345–50
Chandel NS (2017) Mitochondria: back to the future. Nat Rev Mol Cell Biol 19:76. https://doi.org/10.1038/nrm.2017.133
Chaturvedi M, Kaczmarek L (2014) Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49(1):563–573
Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Kanmogne GD (2008) STAT1 signaling modulates HIV-1–induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood 111(4):2062–2072
Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK (2015) Matrix Metalloproteinase-12 induces blood-brain barrier damage after focal cerebral ischemia. Stroke 46(12):3523–3531. https://doi.org/10.1161/strokeaha.115.011031
Chen T, Dai SH, Li X, Luo P, Zhu J, Wang YH, Fei Z, Jiang XF (2018) Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol 14:229–236. https://doi.org/10.1016/j.redox.2017.09.016
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5(4):472–475
Clark IA, Alleva LM, Vissel B (2010) The roles of TNF in brain dysfunction and disease. Pharmacol Ther 128(3):519–548
Cuadrado E, Rosell A, Borrell-Pagès M, García-Bonilla L, Hernández-Guillamon M, Ortega-Aznar A, Montaner J (2009) Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J Cereb Blood Flow Metab 29(2):398–410
Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, Jiang XF (2014) Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med 34(4):1159–1168. https://doi.org/10.3892/ijmm.2014.1876
Dal-Pizzol F, Rojas HA, Dos Santos EM, Vuolo F, Constantino L, Feier G, Pasquali M, Comim CM, Petronilho F, Gelain DP (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood–brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48(1):62–70
de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J (1996) The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 64(1):37–43
Derakhshankhah H, Sajadimajd S, Jafari S, Izadi Z, Sarvari S, Sharifi M, Falahati M, Moakedi F, Muganda WCA, Müller M, Raoufi M, Presley JF (2020) Novel therapeutic strategies for Alzheimer’s disease: Implications from cell-based therapy and nanotherapy. Nanomed Nanotechnol Biol Med 24:102149. https://doi.org/10.1016/j.nano.2020.102149
Dietrich J-B (2002) The adhesion molecule ICAM-1 and its regulation in relation with the blood–brain barrier. J Neuroimmunol 128(1–2):58–68
Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV (2007) Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38(4):1345–1353
Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, Ren X (2015) Mitochondrial crisis in cerebrovascular endothelial cells opens the blood–brain barrier. Stroke 46(6):1681–9
ElAli A, Hermann DM (2012) Liver X receptor activation enhances blood-brain barrier integrity in the ischemic brain and increases the abundance of ATP-binding cassette transporters ABCB1 and ABCC1 on brain capillary cells. Brain Pathol 22(2):175–187. https://doi.org/10.1111/j.1750-3639.2011.00517.x
Elgendy IY, Mahmoud AN, Mansoor H, Mojadidi MK, Bavry AA (2016) Evolution of acute ischemic stroke therapy from lysis to thrombectomy: similar or different to acute myocardial infarction? Int J Cardiol 222:441–447
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364(7):656–665
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G (2014) Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384(9958):1929–1935
Evans A (2016) Timeline: mitochondria. Mol Cell 61(5):790. https://doi.org/10.1016/j.molcel.2016.02.015
Fan Y, Xiong X, Zhang Y, Yan D, Jian Z, Xu B, Zhao H (2016) MKEY, a peptide inhibitor of CXCL4-CCL5 heterodimer formation, protects against stroke in mice. J Am Heart Assoc 5(9):e003615
Fang Z, He Q-W, Li Q, Chen X-L, Baral S, Jin H-J, Zhu Y-Y, Li M, Xia Y-P, Mao L (2016) MicroRNA-150 regulates blood–brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J 30(6):2097–2107
Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M (1997) TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med 3(8):553
Förster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D (2008) Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood–brain barrier. J Physiol 586(7):1937–1949
Fraser PA (2011) The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med 51(5):967–977
Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, Nishimura M, Kataoka H, Hashimoto N, Nozaki K (2008) Tissue inhibitor of metalloproteinases protect blood—brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab 28(10):1674–1685
Galluzzi L, Kepp O, Kroemer G (2012) Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 13(12):780
Gasche Y, Copin J-C, Sugawara T, Fujimura M, Chan PH (2001) Matrix metalloproteinase inhibition prevents oxidative stress-associated blood–brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21(12):1393–1400
Ge X-T, Lei P, Wang H-C, Zhang A-L, Han Z-L, Chen X, Li S-H, Jiang R-C, Kang C-S, Zhang J-N (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 4:6718
Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J (2015) MiR-21 alleviates secondary blood–brain barrier damage after traumatic brain injury in rats. Brain Res 1603:150–157
Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe C-U, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40(5):1849–1857
Geng Y, Li E, Mu Q, Zhang Y, Wei X, Li H, Cheng L, Zhang B (2015) Hydrogen Sulfide Inhalation Decreases Early Blood—Brain Barrier Permeability and Brain Edema Induced by Cardiac Arrest and Resuscitation. J Cereb Blood Flow Metab 35(3):494–500
Giraud M, Cho TH, Nighoghossian N, Maucort-Boulch D, Deiana G, Ostergaard L, Baron JC, Fiehler J, Pedraza S, Derex L, Berthezene Y (2015) Early blood brain barrier changes in acute ischemic stroke: a sequential MRI study. J Neuroimag 25(6):959–963. https://doi.org/10.1111/jon.12225
Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, Jander S (2012) Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol 71(6):743–752
Goldmann EE (1909) Die aussere und innere Sekretion des gesunden und kranken Organismus im Liche der” vitalen Farbung”. Beitr Klin Chir 64:192–265
Grasmick KA, Hu H, Hone EA, Farooqi I, Rellick SL, Simpkins JW, Ren X (2018) Uncoupling of the Electron Transport Chain Compromises Mitochondrial Oxidative Phosphorylation and Exacerbates Stroke Outcomes. J Neuroinfect Dis 9(4). https://doi.org/10.4172/2314-7326.1000283
Graves DT, Jiang Y (1995) Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med 6(2):109–118
Grossmann J (2002) Molecular mechanisms of “detachment-induced apoptosis—Anoikis.” Apoptosis 7(3):247–260
Guan R, Zou W, Dai X, Yu X, Liu H, Chen Q, Teng W (2018) Mitophagy, a potential therapeutic target for stroke. J Biomed Sci 25(1):87. https://doi.org/10.1186/s12929-018-0487-4
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood–brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23(1):87–96
György B, Hung ME, Breakefield XO, Leonard JN (2015) Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol 55:439–464
Hannocks MJ, Zhang X, Gerwien H, Chashchina A, Burmeister M, Korpos E, Song J, Sorokin L (2017) The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. https://doi.org/10.1016/j.matbio.2017.11.007
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. https://doi.org/10.1038/nature18928
Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, Arai K, Rosell A, Lo EH (2018) Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells 36(9):1404–1410
Hernandez-Guillamon M, Martinez‐Saez E, Delgado P, Domingues‐Montanari S, Boada C, Penalba A, Boada M, Pagola J, Maisterra O, Rodriguez‐Luna D (2012) MMP‐2/MMP‐9 plasma level and brain expression in cerebral amyloid angiopathy‐associated hemorrhagic stroke. Brain Pathol 22(2):133–141
Hjort N, Wu O, Ashkanian M, Solling C, Mouridsen K, Christensen S, Gyldensted C, Andersen G, Ostergaard L (2008) MRI detection of early blood-brain barrier disruption: parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke 39(3):1025–1028. https://doi.org/10.1161/STROKEAHA.107.497719
Hone A, Hu E, Sprowls H, Farooqi AS, Grasmick I, Lockman K, Simpkins RP, Ren WJ (2018) Biphasic blood-brain barrier openings after stroke. Neurol Disord Stroke Int 1(3):1–4
Hori S, Ohtsuki S, Hosoya Ki, Nakashima E, Terasaki T (2004) A pericyte-derived angiopoietin‐1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie‐2 activation in vitro. J Neurochem 89(2):503–513
Hu H, Doll DN, Sun J, Lewis SE, Wimsatt JH, Kessler MJ, Simpkins JW, Ren X (2016) Mitochondrial impairment in cerebrovascular endothelial cells is involved in the correlation between body temperature and stroke severity. Aging Dis 7(1):14
Hu H, Hone EA, Provencher EAP, Sprowls SA, Farooqi I, Corbin DR, Sarkar SN, Hollander JM, Lockman PR, Simpkins JW, Ren X (2020) MiR-34a interacts with cytochrome c and shapes stroke outcomes. Sci Rep 10(1):3233. https://doi.org/10.1038/s41598-020-59997-y
Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80(4):844–866
Janzer RC, Raff MC (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325(6101):253
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MV, Chen J, Keep RF, Shi Y (2017) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol
Jiang T, Zhou S, Li X, Song J, An T, Huang X, Ping X, Wang L (2019) MicroRNA-155 induces protection against cerebral ischemia/reperfusion injury through regulation of the Notch pathway in vivo. Exp Ther Med 18(1):605–613. https://doi.org/10.3892/etm.2019.7590
Jin X, Liu J, Liu KJ, Rosenberg GA, Yang Y, Liu W (2013) Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp Neurol 240:9–16
Joy MT, Ben Assayag E, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M, Abduljawad N, Kliper E, Korczyn AD, Thareja NS, Kesner EL, Zhou M, Huang S, Silva TK, Katz N, Bornstein NM, Silva AJ, Shohami E, Carmichael ST (2019) CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 176(5):1143-1157.e1113. https://doi.org/10.1016/j.cell.2019.01.044
Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, Tyagi SC, Tyagi N (2014) Role of microRNA29b in blood–brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. J Cereb Blood Flow Metab 34(7):1212–1222
Kanemoto Y, Nakase H, Akita N, Sakaki T (2002) Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery 51(4):1034–1042
Kassner A, Roberts T, Taylor K, Silver F, Mikulis D (2005) Prediction of hemorrhage in acute ischemic stroke using permeability MR imaging. Am J Neuroradiol 26(9):2213–2217
Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM (2014) Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Transl Stroke Res 5(1):3–16
Kim AS, Nguyen-Huynh M, Johnston SC (2011) A cost–utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke 42(7):2013–2018
Kisler K, Nelson AR, Montagne A, Zlokovic BV (2017) Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18(7):419
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41(2):271–290
Knowland D, Arac A, Sekiguchi Kohei J, Hsu M, Lutz Sarah E, Perrino J, Steinberg Gary K, Barres Ben A, Nimmerjahn A, Agalliu D (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82(3):603–617. https://doi.org/10.1016/j.neuron.2014.03.003
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3):159
Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo J-M, Ford GA (2003) Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 34(11):2543–2548
Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D (2013) Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One 8(2):e56419
Krueger M, Bechmann I, Immig K, Reichenbach A, Härtig W, Michalski D (2015) Blood—brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab 35(2):292–303
Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR (2008) Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 55(3):281–288
Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R (2004) Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 18(6):690–692
Lai CP-K, Breakefield XO (2012) Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol 3:228
Langhauser F, Kraft P, Gob E, Leinweber J, Schuhmann MK, Lorenz K, Gelderblom M, Bittner S, Meuth SG, Wiendl H, Magnus T, Kleinschnitz C (2014) Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke 45(6):1799–1806. https://doi.org/10.1161/strokeaha.114.005000
Larochelle C, Alvarez JI, Prat A (2011) How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett 585(23):3770–3780
Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Klein RS, Diamond MS (2015) Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 7(284):284ra259-284ra259
Lee H, Pienaar IS (2014) Disruption of the blood-brain barrier in Parkinson’s disease: curse or route to a cure. Front Biosci (Landmark Ed) 19:272–280
Liu J, Jin X, Liu KJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood–brain barrier damage in early ischemic stroke stage. J Neurosci 32(9):3044–3057
Liu Y-C, Tsai Y-H, Tang S-C, Liou H-C, Kang K-H, Liou H-H, Jeng J-S, Fu W-M (2018) Cytokine MIF enhances blood-brain barrier permeability: impact for therapy in ischemic stroke. Sci Rep 8(1):1–12
Logsdon AF, Erickson MA, Rhea EM, Salameh TS, Banks WA (2018) Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp Biol Med (Maywood) 243(2):159–165. https://doi.org/10.1177/1535370217743766
Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, Pfizenmaier K, Male DK, Sharrack B, Romero IA (2012) Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol 189(6):3130–9
Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA (2014) MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J 28(6):2551–2565. https://doi.org/10.1096/fj.13-248880
Losy J, Zaremba J (2001) Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke 32(11):2695–2696
Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele EJ, Strayer DS (2011) HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur J Neurosci 34(12):2015–2023
Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Khatri P, Tomsick T, Sharp FR (2009) Mechanical reperfusion is associated with post-ischemic hemorrhage in rat brain. Exp Neurol 216(2):407–412
Malemud CJ (2006) Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci 11(1):1696–1701
Mark KS, Miller DW (1999) Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-α exposure. Life Sci 64(21):1941–1953
McColl BW, Rothwell NJ, Allan SM (2008) Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 28(38):9451–9462. https://doi.org/10.1523/jneurosci.2674-08.2008
McColl BW, Rose N, Robson FH, Rothwell NJ, Lawrence CB (2010) Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab 30(2):267–272. https://doi.org/10.1038/jcbfm.2009.217
Mennicken F, Maki R, de Souza EB, Quirion R (1999) Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci 20(2):73–78
Minagar A, Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler J 9(6):540–549
Mishra R, Singh SK (2013) HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. J Neurosci 33(14):5992–6000
Miyamoto S, Teramoto H, Gutkind JS, Yamada KM (1996) Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol 135(6):1633–1642
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69(3):562–573
Nahirney PC, Reeson P, Brown CE (2016) Ultrastructural analysis of blood–brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J Cereb Blood Flow Metab 36(2):413–425
Neuhaus J, Risau W, Wolburg H (1991) Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculturea. Ann N Y Acad Sci 633(1):578–580
Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y (2010) Tumor necrosis factor-α mediates the blood–brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci 112(2):251–254
Nunnari J, Suomalainen A (2012) Mitochondria: in sickness and in health. Cell 148(6):1145–1159
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485(7397):251
Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1(5):409–417
Ortiz GG, Pacheco-Moises FP, Macias-Islas MA, Flores-Alvarado LJ, Mireles-Ramirez MA, Gonzalez-Renovato ED, Hernandez-Navarro VE, Sanchez-Lopez AL, Alatorre-Jimenez MA (2014) Role of the blood-brain barrier in multiple sclerosis. Arch Med Res 45(8):687–697. https://doi.org/10.1016/j.arcmed.2014.11.013
Paiva KBS, Granjeiro JM (2014) Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys 561:74–87
Peerschke EI, Yin W, Ghebrehiwet B (2010) Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol 47(13):2170–2175
Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM (2015) Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129(2):239–257
Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43(4):348–364
Ramiro L, Simats A, García-Berrocoso T, Montaner J (2018) Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther Adv Neurol Disord 11:1756286418789340
Ransohoff RM (2002) The chemokine system in neuroinflammation: an update. J Infect Dis 186(Supplement_2):S152–S156
Relton JK, Martin D, Thompson RC, Russell DA (1996) Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol 138(2):206–213. https://doi.org/10.1006/exnr.1996.0059
Ren X, Simpkins JW (2015) Deciphering the Blood-Brain Barrier Damage in Stroke: Mitochondrial Mechanism. J Neuroinfectious Dis 6(Suppl 2). https://doi.org/10.4172/2314-7326.S2-e002
Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS, Offner H (2012) Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis 27(1):7–15
Ren X, Engler-Chiurazzi EB, Russell AE, Sarkar SN, Rellick SL, Lewis S, Corbin D, Clapper J, Simpkins JW (2019) MiR-34a and stroke: Assessment of non-modifiable biological risk factors in cerebral ischemia. Neurochem Int 127:73–79. https://doi.org/10.1016/j.neuint.2018.10.019
Richard S, Lagerstedt L, Burkhard PR, Debouverie M, Turck N, Sanchez J-C (2015) E-selectin and vascular cell adhesion molecule-1 as biomarkers of 3-month outcome in cerebrovascular diseases. J Inflamm 12(1):61
Ridker PM (2018) Interleukin-1 inhibition and ischaemic stroke: has the time for a major outcomes trial arrived? Eur Heart J 39(38):3518–3520. https://doi.org/10.1093/eurheartj/ehy360
Rochfort KD, Cummins PM (2015) The blood–brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans 43(4):702–706
Rolfe D, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77(3):731–758
Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG (1992) TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res 576(2):203–207
Rosenberg G, Estrada E, Dencoff J (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29(10):2189–2195
Salameh TS, Shah GN, Price TO, Hayden MR, Banks WA (2016) Blood–brain barrier disruption and neurovascular unit dysfunction in diabetic mice: protection with the mitochondrial carbonic anhydrase inhibitor topiramate. J Pharmacol Exp Ther 359(3):452–459
Scalzo F, Alger JR, Hu X, Saver JL, Dani KA, Muir KW, Demchuk AM, Coutts SB, Luby M, Warach S, Liebeskind DS (2013) Multi-center prediction of hemorrhagic transformation in acute ischemic stroke using permeability imaging features. Magn Reson Imaging 31(6):961–969. https://doi.org/10.1016/j.mri.2013.03.013
Shimamura N, Matchett G, Solaroglu I, Tsubokawa T, Ohkuma H, Zhang J (2006) Inhibition of integrin alphavbeta3 reduces blood-brain barrier breakdown in focal ischemia in rats. J Neurosci Res 84(8):1837–1847. https://doi.org/10.1002/jnr.21073
Smith C Leukocyte-endothelial cell interactions. In: Seminars in hematology, 1993. vol 4 Suppl 4. pp 45–53; discussion 54 – 45
Sobowale OA, Parry-Jones AR, Smith CJ, Tyrrell PJ, Rothwell NJ, Allan SM (2016) Interleukin-1 in stroke: from bench to bedside. Stroke 47(8):2160–2167. https://doi.org/10.1161/strokeaha.115.010001
Solé S, Petegnief V, Gorina R, Chamorro Á, Planas AM (2004) Activation of matrix metalloproteinase-3 and agrin cleavage in cerebral ischemia/reperfusion. J Neuropathol Exp Neurol 63(4):338–349
Song L, Pachter JS (2004) Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc Res 67(1):78–89
Sørensen SS, Nygaard A-B, Nielsen M-Y, Jensen K, Christensen T (2014) miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res 5(6):711–718
Spera PA, Ellison JA, Feuerstein GZ, Barone FC (1998) IL-10 reduces rat brain injury following focal stroke. Neurosci Lett 251(3):189–192
Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV (2005) Monocyte chemoattractant protein-1 regulation of blood–brain barrier permeability. J Cereb Blood Flow Metab 25(5):593–606
Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV (2006) Protein kinase C-alpha: Rhoa cross talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem 281(13):8379–88
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17(1):463–516
Sun N, Youle RJ, Finkel T (2016) The mitochondrial basis of aging. Mol Cell 61(5):654–666
Suofu Y, Wang X, He Y, Li F, Zhang Y, Carlisle DL, Friedlander RM (2020) Mir-155 knockout protects against ischemia/reperfusion-induced brain injury and hemorrhagic transformation. Neuroreport 31(3):235–239. https://doi.org/10.1097/WNR.0000000000001382
Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS (2010) Non-coding RNAs: regulators of disease. J Pathol 220(2):126–139. https://doi.org/10.1002/path.2638
Tan S, Shan Y, Lin Y, Liao S, Zhang B, Zeng Q, Wang Y, Deng Z, Chen C, Hu X (2019) Neutralization of interleukin-9 ameliorates experimental stroke by repairing the blood–brain barrier via down-regulation of astrocyte-derived vascular endothelial growth factor-A. FASEB J 33(3):4376–4387
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T (2015) Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6:6716–6716. https://doi.org/10.1038/ncomms7716
Vemuganti R, Dempsey RJ, Bowen KK (2004) Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke 35(1):179–184
Vishnu VY, Padma Srivastava MV (2019) Innovations in acute stroke reperfusion strategies. Ann Indian Acad Neurol 22(1):6–12. https://doi.org/10.4103/aian.AIAN_263_18
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184(1–2):53–68
Wang Y, Wang M-D, Xia Y-P, Gao Y, Zhu Y-Y, Chen S-C, Mao L, He Q-W, Yue Z-Y, Hu B (2017) MicroRNA-130a regulates cerebral ischemia–induced blood–brain barrier permeability by targeting Homeobox A5. FASEB J 32(2):935–944
Weiss N, Miller F, Cazaubon S, Couraud P-O (2009) The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta (BBA) - Biomembr 1788(4):842–857
Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G (2016) Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 15(9):925–933
Wing SC, Markus HS (2019) Interpreting CT perfusion in stroke. Pract Neurol 19(2):136–142
Wu J, Zhao D, Wu S, Wang D (2015a) Ang-(1–7) exerts protective role in blood–brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol 748:30–36
Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, Chan M, Zhou X, Qasem S, Pochampally R (2015) Roles of the cyclooxygenase 2-matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem 290(15):9842–54
Xia Y, Cai W, Thomson AW, Hu X (2016) Regulatory T cell therapy for ischemic stroke: how far from clinical translation? Transl Stroke Res 7(5):415–419. https://doi.org/10.1007/s12975-016-0476-4
Xiong X-Y, Liu L, Yang Q-W (2016) Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 142:23–44
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27(4):697–709
Yang Y, Rosenberg GA (2015) Matrix metalloproteinases as therapeutic targets for stroke. Brain Res 1623:30–38
Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y (2015) Attenuation of acute stroke injury in rat brain by minocycline promotes blood–brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflamm 12(1):26
Yang X, Tang X, Sun P, Shi Y, Liu K, Hassan SH, Stetler RA, Chen J, Yin KJ (2017) MicroRNA-15a/16 – 1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke 48(7):1941–1947. https://doi.org/10.1161/strokeaha.117.017284
Yao Y, Tsirka SE (2014) Monocyte chemoattractant protein-1 and the blood–brain barrier. Cell Mol Life Sci 71(4):683–697
Yao X, Wang Y, Zhang D (2018) microRNA-21 confers neuroprotection against cerebral ischemia-reperfusion injury and alleviates blood-brain barrier disruption in rats via the MAPK signaling pathway. J Mol Neurosci 65(1):43–53
Yin K-J, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE (2010) Peroxisome proliferator-activated receptor δ regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci 30(18):6398–6408
Yin K-J, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE (2010b) miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 38(1):17–26
Zhang L, Chopp M, Jia L, Cui Y, Lu M, Zhang ZG (2009) Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J Cereb Blood Flow Metab 29(11):1816–1824
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163(5):1064–1078
Zhao Z, Ong LK, Johnson S, Nilsson M, Walker FR (2017) Chronic stress induced disruption of the peri-infarct neurovascular unit following experimentally induced photothrombotic stroke. J Cereb Blood Flow Metab 37(12):3709–3724
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201
Zuo X, Lu J, Manaenko A, Qi X, Tang J, Mei Q, Xia Y, Hu Q (2019) MicroRNA-132 attenuates cerebral injury by protecting blood-brain-barrier in MCAO mice. Exp Neurol 316:12–19. https://doi.org/10.1016/j.expneurol.2019.03.017
Funding
The work was supported by AHA (16SDG31170008 to XR), NSF (1008182R to XR), WVCTSI (NIH/NIGMS U54GM104942 to XR), WVU Bridge Funding Grant (to XR) and NIH (P20 GM109098 to JWS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarvari, S., Moakedi, F., Hone, E. et al. Mechanisms in blood-brain barrier opening and metabolism-challenged cerebrovascular ischemia with emphasis on ischemic stroke. Metab Brain Dis 35, 851–868 (2020). https://doi.org/10.1007/s11011-020-00573-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00573-8