Abstract
Purpose
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related mortality world-wide. Recently, a number of circular RNAs (circRNAs) has been found to be differentially expressed in human NSCLCs, correlating with clinico-pathological features. As yet, the expression and potential role of circRNA BIRC6 (circBIRC6) in NSCLC have not been studied.
Methods
Expression of circBIRC6 and its target microRNA-145 (miR-145) in human NSCLC cells and tissues was assessed using qRT-PCR. In vitro genetic strategies were used to exogenously alter circBIRC6 and miR-145 expression. Their impact on in vitro and in vivo NSCLC cell behavior was studied.
Results
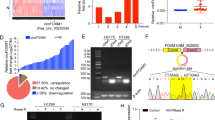
We found that circBIRC6 was upregulated in primary human NSCLC tissues and NSCLC cells, whereas its potential target, miR-145, was downregulated. In A549 NSCLC cells and primary human NSCLC cells, shRNA-induced silencing of circBIRC6 potently inhibited their growth, proliferation, migration and invasion. Conversely, we found that exogenous overexpression of circBIRC6 promoted these characteristics. Using RNA immunoprecipitation (RIP) in A549 cells, we found that Argonaute 2 (Ago2) immunoprecipitated together with both circBIRC6 and miR-145. Additional studies revealed that the miR-145 level increased after circBIRC6 silencing in A549 cells, but decreased after circBIRC6 overexpression. Of note, we found that the circBIRC6 silencing-induced anti-A549 activity could be attenuated by a miR-145 inhibitor. Lastly, we found that circBIRC6 silencing inhibited the growth of NSCLC xenografts in severe combined immunodeficient mice.
Conclusions
From our data we conclude that circBIRC6 overexpression promotes NSCLC cell progression, possibly by sponging miR-145.





Similar content being viewed by others
Availability of supporting data
All data generated during this study are included in this article.
Abbreviations
- Ago2:
-
Argonaute 2
- circRNA:
-
circular RNA
- circBIRC6:
-
circRNA BIRC6
- ECL:
-
enhanced chemiluminescence
- EMT:
-
epithelial-mesenchymal transition
- FSCN1:
-
fascin1
- FBS:
-
fetal bovine serum
- mTOR:
-
mammalian target of rapamycin
- miR-145:
-
microRNA-145
- ncRNA:
-
non-coding RNA
- NSCLC:
-
non-small cell lung cancer
- PI:
-
propidium iodide
- RIP:
-
RNA immunoprecipitation
- SCID:
-
severe combined immunodeficient
References
R. Rosell, N. Karachaliou, Lung cancer in 2014: optimizing lung cancer treatment approaches. Nat. Rev. Clin. Oncol. 12, 75–76 (2015)
J.W. Neal, J.F. Gainor, A.T. Shaw, Developing biomarker-specific end points in lung cancer clinical trials. Nat. Rev. Clin. Oncol. 12, 135–146 (2015)
R.L. Keith, Y.E. Miller, Lung cancer chemoprevention: current status and future prospects. Nat. Rev. Clin. Oncol. 10, 334–343 (2013)
L. Cortes-Dericks, D. Galetta, The therapeutic potential of mesenchymal stem cells in lung cancer: benefits, risks and challenges. Cell. Oncol. 42, 727–738 (2019)
J. Huang, Y. Li, Z. Lu, Y. Che, S. Sun, S. Mao, Y. Lei, R. Zang, N. Li, S. Zheng, C. Liu, X. Wang, N. Sun, J. He, Analysis of functional hub genes identifies CDC45 as an oncogene in non-small cell lung cancer - a short report. Cell. Oncol. 42, 571–578 (2019)
R. Siegel, J. Ma, Z. Zou, A. Jemal, Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014)
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 (2015)
W. Chen, R. Zheng, P.D. Baade, S. Zhang, H. Zeng, F. Bray, A. Jemal, X.Q. Yu, J. He, Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 (2016)
K.K. Ebbesen, T.B. Hansen, J. Kjems, Insights into circular RNA biology. RNA Biol. 14, 1035–1045 (2017)
S. Qu, X. Yang, X. Li, J. Wang, Y. Gao, R. Shang, W. Sun, K. Dou, H. Li, Circular RNA: a new star of noncoding RNAs. Cancer Lett. 365, 141–148 (2015)
X. Chen, R. Mao, W. Su, X. Yang, Q. Geng, C. Guo, Z. Wang, J. Wang, L.A. Kresty, D.G. Beer, A.C. Chang, G. Chen, Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKalpha signaling in STK11 mutant lung cancer. Autophagy 16, 659–671 (2019)
S. Wei, Y. Zheng, Y. Jiang, X. Li, J. Geng, Y. Shen, Q. Li, X. Wang, C. Zhao, Y. Chen, Z. Qian, J. Zhou, W. Li, The circRNA circPTPRA suppresses epithelial-mesenchymal transitioning and metastasis of NSCLC cells by sponging miR-96-5p. EBioMedicine 44, 182–193 (2019)
F. de Fraipont, S. Gazzeri, W.C. Cho, B. Eymin, Circular RNAs and RNA splice variants as biomarkers for prognosis and therapeutic response in the liquid biopsies of lung cancer patients. Front. Genet. 10, 390 (2019)
K. Wu, X. Liao, Y. Gong, J. He, J.K. Zhou, S. Tan, W. Pu, C. Huang, Y.Q. Wei, Y. Peng, Circular RNA F-circSR derived from SLC34A2-ROS1 fusion gene promotes cell migration in non-small cell lung cancer. Mol. Cancer 18, 98 (2019)
L. Chen, A. Nan, N. Zhang, Y. Jia, X. Li, Y. Ling, J. Dai, S. Zhang, Q. Yang, Y. Yi, Y. Jiang, Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol. Cancer 18, 13 (2019)
C.Y. Yu, T.C. Li, Y.Y. Wu, C.H. Yeh, W. Chiang, C.Y. Chuang, H.C. Kuo, The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat. Commun. 8, 1149 (2017)
H. Yu, Y. Chen, P. Jiang, Circular RNA HIPK3 exerts oncogenic properties through suppression of miR-124 in lung cancer. Biochem. Biophys. Res. Commun. 506, 455–462 (2018)
B. Zhang, H.Y. Lu, Y.H. Xia, A.G. Jiang, Y.X. Lv, Long non-coding RNA EPIC1 promotes human lung cancer cell growth. Biochem. Biophys. Res. Commun. 503, 1342–1348 (2018)
F. Wu, F. Liu, L. Dong, H. Yang, X. He, L. Li, L. Zhao, S. Jin, G. Li, miR-1273 g silences MAGEA3/6 to inhibit human colorectal cancer cell growth via activation of AMPK signaling. Cancer Lett. 435, 1–9 (2018)
T. Chiyomaru, H. Enokida, S. Tatarano, K. Kawahara, Y. Uchida, K. Nishiyama, L. Fujimura, N. Kikkawa, N. Seki, M. Nakagawa, miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer 102, 883–891 (2010)
Q. Xu, L.Z. Liu, X. Qian, Q. Chen, Y. Jiang, D. Li, L. Lai, B.H. Jiang, MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 40, 761–774 (2012)
A. Quintanal-Villalonga, S. Molina-Pinelo, Epigenetics of lung cancer: a translational perspective. Cell. Oncol. 42, 739–756 (2019)
S. Ebrahimi, S.I. Hashemy, MicroRNA-mediated redox regulation modulates therapy resistance in cancer cells: clinical perspectives. Cell. Oncol. 42, 131–141 (2019)
R. Castro-Oropeza, J. Melendez-Zajgla, V. Maldonado, K. Vazquez-Santillan, The emerging role of lncRNAs in the regulation of cancer stem cells. Cell. Oncol. 41, 585–603 (2018)
L. Li, X. Zhang, Q. Liu, H. Yin, Y. Diao, Z. Zhang, Y. Wang, Y. Gao, X. Ren, J. Li, D. Cui, Y. Lu, H. Liu, Emerging role of HOX genes and their related long noncoding RNAs in lung cancer. Crit. Rev. Oncol. Hematol. 139, 1–6 (2019)
H. Yu, L. Xu, Z. Liu, B. Guo, Z. Han, H. Xin, Circ_MDM2_000139, Circ_ATF2_001418, Circ_CDC25C_002079, and Circ_BIRC6_001271 are involved in the functions of XAV939 in non-small cell lung cancer. Can. Respir. J. 2019, 9107806 (2019)
M. Jin, J. Ren, M. Luo, Z. You, Y. Fang, Y. Han, G. Li, H. Liu, Long noncoding RNA JPX correlates with poor prognosis and tumor progression in non-small cell lung cancer by interacting with miR-145-5p and CCND2. Carcinogenesis 2019. https://doi.org/10.1093/carcin/bgz125 (2019)
Q. Liu, J. Chen, B. Wang, Y. Zheng, Y. Wan, Y. Wang, L. Zhou, S. Liu, G. Li, Y. Yan, miR-145 modulates epithelial-mesenchymal transition and invasion by targeting ZEB2 in non-small cell lung cancer cell lines. J. Cell. Biochem. https://doi.org/10.1002/jcb.28126 (2018)
Y. Pan, C. Ye, Q. Tian, S. Yan, X. Zeng, C. Xiao, L. Wang, H. Wang, miR-145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL-2 ratio and the caspase-3 cascade. Oncol. Lett. 15, 4337–4343 (2018)
Y. Liu, X. Chen, J. Yao, J. Kang, Circular RNA ACR relieves high glucose-aroused RSC96 cell apoptosis and autophagy via declining microRNA-145-3p. J. Cell. Biochem. https://doi.org/10.1002/jcb.29568 (2019)
X. Yang, P. Lei, Y. Huang, Z. Zhang, Y. Zhang, MicroRNA-133b inhibits the migration and invasion of non small cell lung cancer cells via targeting FSCN1. Oncol. Lett. 12, 3619–3625 (2016)
J. Zhao, Y. Zhou, Z. Zhang, F. Tian, N. Ma, T. Liu, Z. Gu, Y. Wang, Upregulated fascin1 in non-small cell lung cancer promotes the migration and invasiveness, but not proliferation. Cancer Lett. 290, 238–247 (2010)
Y. Ma, L.M. Machesky, Fascin1 in carcinomas: Its regulation and prognostic value. Int. J. Cancer 137, 2534–2544 (2014)
M.B. Chen, M.X. Wei, J.Y. Han, X.Y. Wu, C. Li, J. Wang, W. Shen, P.H. Lu, MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression in HT-29 colorectal cancer. Cell. Signal. 26, 102–109 (2014)
R.A. Saxton, D.M. Sabatini, mTOR signaling in growth, metabolism, and disease. Cell. 168, 960–976 (2017)
H.H. Vestergaard, M.R. Christensen, U.N. Lassen, A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol. 57, 176–186 (2018)
D.M. Sabatini, mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6, 729–734 (2006)
Acknowledgements
We thank Dr. Jiang from Nanjing Medical University for manuscript editing and study design.
Authors’ information
Not applicable.
Funding
This project was supported by a Medicine and Health Grant from Wenzhou Municipal Science and Technology Bureau (Y20180213). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All listed authors participated in designing the study, performing the experiments and the statistical analyses, and writing the manuscript. All authors have read and approved the final version.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Wenzhou Medical University.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, H., Zhao, M., Zhao, L. et al. CircRNA BIRC6 promotes non-small cell lung cancer cell progression by sponging microRNA-145. Cell Oncol. 43, 477–488 (2020). https://doi.org/10.1007/s13402-020-00503-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00503-x