Abstract
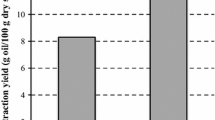
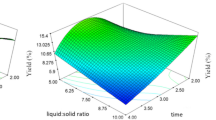
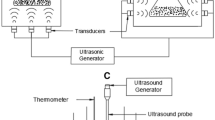
Date seed oil is a good source of natural antioxidants due to the presence of phenols, tocopherols, and sterols. The present research is the first study on the extraction of date seed oil by combining the ultrasound-assisted aqueous enzymatic extraction method and the cooking pretreatment. In this study, the effects of two independent variables namely the sample to solvent ratio (at six levels of 1: 2, 1: 3, 1: 4, 1: 6, 1: 8 and 1:10) and the cooking time (at four levels of 0, 20, 40 and 60) were investigated on dependent variables namely the extraction yield, total phenolic compound, peroxide value, and acid value of date seed oil. The results indicated that optimum conditions for extraction of date seed oil were sample to solvent ratio of 1: 2 and the cooking time of 40 min. Furthermore, the Scanning Electron Microscopy images of date seed powder after selective treatments indicated the destruction and fragmentation of residual pulp structures by increasing the cooking time. In general, results of the present study indicated that it was important to select the appropriate sample to solvent ratio and implement the pretreatment of cooking to extract oil with high yield and quality. Combination of the ultrasound-assisted aqueous enzymatic extraction method and the cooking pretreatment can be proposed as a new extraction method in the oil industry. This new extraction method can improve the oil extraction efficiency and the quality of extracted oil.






Similar content being viewed by others
References
Food and Agriculture Organization of the United Nations,. Faostat.Fao.Org/Site/339/Default.Aspx. 2010
Ashraf Z, Hamidi-Esfahani Z (2011) Date and date processing: a review. Food Rev Int 27:101–133
Abbès F, Bouaziz MA, Blecker C, Masmoudi M et al (2011) Date syrup: effect of hydrolytic enzymes (pectinase/cellulase) on physico-chemical characteristics, sensory and functional properties. LWT - Food Sci. Technol. 44:1827–1834
Aziz H, Aslan A, Ramin T, Ata KK et al (2015) Evaluation of physicochemical, rheological, microbial and sensory properties of canned date. Iran J Nutr Sci Food Technol 10:95–102
Razavi SMA, Karazhiyan H (2012) Rheological and textural characteristics of date paste. Int J Food Prop 15:281–291
Khatchadourian H, Sawaya W, Ayaz M, Al-Mohammad MM (1987) Processing date varieties into pickles. Int J Food Sci Technol 22:243–247
Roukas T, Kotzekidou P (1997) Pretreatment of date syrup to increase citric acid production. Enzym Microb Technol 21:273–276
Louhichi B, Belgaib J, Benamor H, Hajji N (2013) Production of bio-ethanol from three varieties of dates. Renew Energy 51:170–174
Al-Farsi MA, Lee CY (2008) Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem 108:977–985
Habib HM, Kamal H, Ibrahim WH, Dhaheri AS (2013) Al, carotenoids, fat soluble vitamins and fatty acid profiles of 18 varieties of date seed oil. Ind Crop Prod 42:567–572
Habib HM, Platat C, Meudec E, Cheynier V et al (2014) Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and quantification by using UPLC-DAD-ESI-MS. J Sci Food Agric 94:1084–1089
Abdelaziz DHA, Ali SA (2014) The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. J Ethnopharmacol 155:736–743
Devshony S, Eteshola E, Shani A (1992) Characteristics and some potential applications of date palm (Phoenix dactylifera L.) seeds and seed oil. J Am Oil Chem Soc 69:595–597
Besbes S, Blecker C, Deroanne C, Drira N-E et al (2004) Date seeds: chemical composition and characteristic profiles of the lipid fraction. Food Chem 84:577–584
Al-Shahib W, Marshall RJ (2003) Fatty acid content of the seeds from 14 varieties of date palm Phoenix dactylifera L. Int J Food Sci Technol 38:709–712
Corso MP, Fagundes-Klen MR, Silva EA, Cardozo Filho L et al (2010) Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J Supercrit Fluids 52:56–61
Li Y, Fabiano-Tixier AS, Tomao V, Cravotto G et al (2013) Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason Sonochem 20:12–18
Chen F, Zhang Q, Gu H, Yang L (2016) An approach for extraction of kernel oil from Pinus pumila using homogenate-circulating ultrasound in combination with an aqueous enzymatic process and evaluation of its antioxidant activity. J Chromatogr A 1471:68–79
Latif S, Diosady LL, Anwar F (2008) Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur J Lipid Sci Technol 110:887–892
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzym Microb Technol 19:402–420
Khoei M, Chekin F (2016) The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem 194:503–507
Shalmashi A, Golmohammad F, Sadr ZB (2017) Multi-satge solvent extraction of soybean isoflavones. Innov Food Technol 4:93–107
Chen J, Yu X, Geng Q, Li M (2018) Combination of span 20 and pH-assisted walnut oil extraction during aqueous extraction process. LWT - Food Sci Technol 91:477–483
Zhang SB, Wang Z, Xu SY (2007) Optimization of the aqueous enzymatic extraction of rapeseed oil and protein hydrolysates. J Am Oil Chem Soc 84:97–105
Zhang Y, Li S, Yin C, Jiang D et al (2012) Response surface optimisation of aqueous enzymatic oil extraction from bayberry (Myrica rubra) kernels. Food Chem 135:304–308
Liu Y, Gong G, Zhang J, Jia S et al (2014) Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr Polym 110:278–284
Haji Heidari S, Taghian Dinani S (2018) The study of ultrasound-assisted enzymatic extraction of oil from peanut seeds using response surface methodology. Eur J Lipid Sci Technol 120:1700252
Bakhshabadi H, Rostami M, Moghimi M, Bojmehrani A et al (2017) Optimizing the operating parameters of cooker during oil extraction and production of sunflower meal on an industrial scale. Iran Food Sci Technol Res J 13:27–37
Shirsath SR, Sonawane SH, Gogate PR (2012) Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem Eng Process Process Intensif 53:10–23
Singh KK, Wiesenborn DP, Tostenson K, Kangas N (2002) Influence of moisture content and cooking on screw pressing of crambe seed. JAOCS, J. Am. Oil Chem. Soc. 79:165–170
Shah S, Aparna S, M.N., G., (2005) Extraction of oil from Jatropha curcas L. seed kernels by combination of ultrasonic and aqueous enzymatic oil extraction. Bioresour Technol 96:121–123
Jiao J, Li Z-G, Gai Q-Y, Li X-J et al (2014) Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem 147:17–24
Jiang X, Chang M, Jin Q, Wang X (2014) Effect of ultrasound treatment on oil recovery from soybean gum by using phospholipase C. J Clean Prod 69:237–242
Sharma A, Khare SK, Gupta MN (2002) Enzyme-assisted aqueous extraction of peanut oil. J Am Oil Chem Soc 79:215–218
Ghasemi YZ, Taghian Dinani S (2005) Optimization of ultrasound-assisted enzymatic extraction of walnut kernel oil using response surface methodology. J Food Process Eng e12696
Tian Y, Xu Z, Zheng B, Martin Lo Y (2013) Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason Sonochem 20:202–208
Shahram H, Dinani ST, Amouheydari M (2018) Effects of pectinase concentration, ultrasonic time, and pH of an ultrasonic-assisted enzymatic process on extraction of phenolic compounds from orange processing waste. J Food Meas Charact. 13:487–498
ISIRI, Institute of Standards and Industrial Research of Iran. 1998, 4178
Janporn S, Ho CT, Chavasit V, Pan MH et al (2015) Physicochemical properties of Terminalia catappa seed oil as a novel dietary lipid source. J Food Drug Anal 23:201–209
Rahimpour S, Taghian S (2018) Lycopene extraction from tomato processing waste using ultrasound and cell-wall degrading enzymes. J Food Meas Charact 12:2394–2403
Pinelo M, Rubilar M, Jerez M, Sineiro J et al (2005) Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem 53:2111–2117
Rahnemoon P, Sarabi Jamab M, Javanmard Dakheli M, Bostan A (2107) Evaluation of extraction conditions on phenolic compounds and antimicrobial properties of pomegranate (Punica granatum) peels. Food Sci Technol 65:51–62
Oderinde RA, Ajayi IA, Adewuyi A (2009) Characterization of seed and seed oil of Hura crepitans and the kinetics of degradation of the oil during heating. Electron J Environ Agric Food Chem 8:201–208
Hassan Fatemi, Food chemistry, 2017
Lu X, Ross CF, Powers JR, Aston DE et al (2011) Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated Total reflectance–Fourier transformed infrared spectroscopy. J Agric Food Chem 59:5215–5221
Chavan JJ, Ghadage DM, Kshirsagar PR, Kudale SS (2018) Optimization of extraction techniques and RP-HPLC analysis of antidiabetic and anticancer drug mangiferin from roots of saptarangi (Salacia chinensis L.). J. Liq. Chromatogr. Relat. Technol. 38:963–969
Donald L, P., Lampman, Gary M., George S, K., R.vyvyan, J., Introduction to spectroscopy, 2008
Ye J, Feng L, Xiong J, Xiong Y (2011) Ultrasound-assisted extraction of corn carotenoids in ethanol. Int J Food Sci Technol 46:2131–2136
Ghafoor K (2009) Optimization of ultrasound assisted extraction of phenolic compounds and antioxidants from grape peel through response surface methodology. J Korean Soc Appl Biol Chem 52:295–300
Wiesenborn D, Doddapaneni R, Tostenson K, Kangas N (2001) Cooking indices to predict screw-press performance for crambe seed. J Am Oil Chem Soc 78:467–471
Lee YC, Oh SW, Chang J, Kim IH (2004) Chemical composition and oxidative stability of safflower oil prepared from safflower seed roasted with different temperatures. Food Chem 84:1–6
Mirheli M, Taghian Dinani S (2018) Extraction of β-carotene pigment from carrot processing waste using ultrasonic-shaking incubation method. J. Food Meas. Charact. 12:1818–1828
Khalili F, Taghian Dinani S (2018) Extraction of phenolic compounds from olive-waste cake using ultrasonic process. J Food Meas Charact 12:974–981
Kamil MM, Mohamed GF, Shaheen MS (2011) Fourier Transformer Infrared Spectroscopy for Quality Assurance of Tomato Products. J Am Sci 7:559–572
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Soudabeh Amigh and Somayeh Taghian Dinani declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amigh, S., Taghian Dinani, S. Combination of ultrasound-assisted aqueous enzymatic extraction and cooking pretreatment for date seed oil recovery. Heat Mass Transfer 56, 2345–2354 (2020). https://doi.org/10.1007/s00231-020-02865-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-020-02865-2