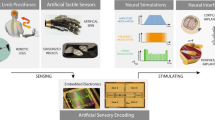
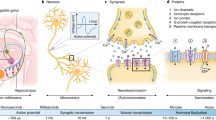
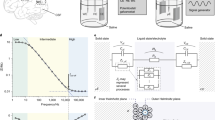
Abstract
Devices such as keyboards and touchscreens allow humans to communicate with machines. Neural interfaces, which can provide a direct, electrical bridge between analogue nervous systems and digital man-made systems, could provide a more efficient route to future information exchange. Here we review the development of electronic neural interfaces. The interfaces typically consist of three modules — a tissue interface, a sensing interface, and a neural signal processing unit — and based on technical milestones in the development of the electronic sensing interface, we group and analyse the interfaces in four generations: the patch clamp technique, multi-channel neural interfaces, implantable/wearable neural interfaces and integrated neural interfaces. We also consider key circuit and system challenges in the design of neural interfaces and explore the opportunities that arise with the latest technology
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Galvani, L. De Viribus Electricitatis In Motu Musculari Commentarius Bologna (Academy of Sciences, 1791).
Marmont, G. Studies on the axon membrane. I. A new method. J. Cell. Compar. Physiol. 34, 351–382 (1949).
Hodgkin, A. L. & Huxley, A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo. J. Physiol. 116, 449–472 (1952).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Neher, E. & Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802 (1976).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg. Arch. 391, 85–100 (1981).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev Neurosci. 13, 407–420 (2012).
Konerding, W., Froriep, U., Kral, A. & Baumhoff, P. New thin-film surface electrode array enables brain mapping with high spatial acuity in rodents. Sci. Rep. 8, 3825 (2018).
Lopez-Gordo, M., Sanchez-Morillo, D. & Valle, F. Dry EEG electrodes. Sensors 14, 12847–12870 (2014).
Xu, J., Mitra, S., Van Hoof, C., Yazicioglu, R. F. & Makinwa, K. A. Active electrodes for wearable EEG acquisition: review and electronics design methodology. IEEE Rev. Biomed. Eng. 10, 187–198 (2017).
Klem, G. H., Lüders, H. O., Jasper, H. & Elger, C. et al. The ten-twenty electrode system of the international federation. Electroen. Clin. Neurophysiol. 52, 3–6 (1999).
Jurcak, V., Tsuzuki, D. & Dan, I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage 34, 1600–1611 (2007).
Leuthardt, E. C., Schalk, G., Wolpaw, J. R., Ojemann, J. G. & Moran, D. W. A brain–computer interface using electrocorticographic signals in humans. J. Neural Eng. 1, 63–71 (2004).
Tolstosheeva, E. et al. A multi-channel, flex-rigid ECoG microelectrode array for visual cortical interfacing. Sensors 15, 832–854 (2015).
Blau, A. et al. Flexible, all-polymer microelectrode arrays for the capture of cardiac and neuronal signals. Biomaterials 32, 1778–1786 (2011).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575–1575 (2013).
Escabí, M. A. et al. A high-density, high-channel count, multiplexed µECoG array for auditory-cortex recordings. J. Neurophysiol. 112, 1566–1583 (2014).
Lehew, G. & Nicolelis, M. A. in Methods for Neural Ensemble Recordings 2nd edn (ed. Nicolelis, M. A.) Ch. 1 (Taylor & Francis, 2008).
Nicolelis, M. A. et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl Acad. Sci. USA 100, 11041–11046 (2003).
Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 (2004).
Obaid, A. M. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Preprint at https://www.biorxiv.org/content/10.1101/573295v1 (2019).
Wise, K. D., Anderson, D., Hetke, J., Kipke, D. & Najafi, K. Wireless implantable microsystems: high-density electronic interfaces to the nervous system. Proc. IEEE 92, 76–97 (2004).
Rousche, P. J. & Normann, R. A. Chronic recording capability of the Utah intracortical electrode array in cat sensory cortex. J. Neurosci. Meth. 82, 1–15 (1998).
Abidian, M. R. & Martin, D. C. Multifunctional nanobiomaterials for neural interfaces. Adv. Func. Mater. 19, 573–585 (2009).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31 (2019).
Hanson, T. L., Diaz-Botia, C. A., Kharazia, V., Maharbiz, M. M. & Sabes, P. N. The “sewing machine” for minimally invasive neural recording. Preprint at https://www.biorxiv.org/content/10.1101/578542v1 (2019).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Patel, P. R. et al. Insertion of linear 8.4 µm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 12, 046009 (2015).
Massey, T. L. et al. A high-density carbon fiber neural recording array technology. J. Neural Eng. 16, 016024 (2019).
Lawrence, S. M., Dhillon, G. S. & Horch, K. W. Fabrication and characteristics of an implantable, polymer-based, intrafascicular electrode. J.Nneurosci. Meth. 131, 9–26 (2003).
Boretius, T. et al. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 26, 62–69 (2010).
Raspopovic, S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19–222ra19 (2014).
Akin, T., Najafi, K., Smoke, R. H. & Bradley, R. M. A micromachined silicon sieve electrode for nerve regeneration applications. IEEE Trans. Biomed. Eng. 41, 305–313 (1994).
Nerve Cuff Electrodes. MicroProbes for Life Science (2020); https://microprobes.com/products/peripheral-electrodes/nerve-cuff
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Merrill, D. R., Bikson, M. & Jefferys, J. G. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Meth. 141, 171–198 (2005).
Van Dongen, M. & Serdijn, W. Design of Efficient and Safe Neural Stimulators (Springer, 2016).
Paulus, W. Transcranial electrical stimulation (tES–tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21, 602–617 (2011).
Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J. Neurosurg. Spine 100, 254–267 (2004).
Kringelbach, M. L., Jenkinson, N., Owen, S. L. & Aziz, T. Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Groves, D. A. & Brown, V. J. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 29, 493–500 (2005).
Hallett, M. Transcranial magnetic stimulation: a primer. Neuron 55, 187–199 (2007).
Luo, D., Zhang, M. & Wang, Z. A low-noise chopper amplifier designed for multi-channel neural signal acquisition. IEEE J. Solid-State Circ. 54, 2255–2265 (2019).
Yaul, F. M. & Chandrakasan, A. P. A sub-µW 36nV/√Hz chopper amplifier for sensors using a noise-efficient inverter-based 0.2V-supply input stage. In IEEE Int. Solid-State Circuits Conference (ISSCC) 94–95 (IEEE, 2016).
Johnson, B. C. et al. An implantable 700µW 64-channel neuromodulation IC for simultaneous recording and stimulation with rapid artifact recovery. In 2017 Symposium on VLSI Circuits C48–C49 (IEEE, 2017).
Kim, C. et al. A 92dB dynamic range sub-μV rms-noise 0.8µW/ch neural-recording ADC array with predictive digital autoranging. In 2018 IEEE Int. Solid-State Circuits Conference (ISSCC) 470–472 (IEEE, 2018).
Chandrakumar, H. & Markovic, D. A 15.2-ENOB continuous-time ΔΣ ADC for a 7.3 µW 200 mV pp-linear-input-range neural recording front-end. In 2018 IEEE Int. Solid-State Circuits Conference (ISSCC) 232–234 (IEEE, 2018).
Rozgić, D. et al. A 0.338cm3, artifact-free, 64-contact neuromodulation platform for simultaneous stimulation and sensing. IEEE Trans. Biomed. Circ. Syst. 13, 38–55 (2018).
Ha, U. et al. An EEG-NIRS multimodal SoC for accurate anesthesia depth monitoring. IEEE J. Solid-State Circ. 53, 1830–1843 (2018).
Lee, S. et al. A 110dB-CMRR 100dB-PSRR multi-channel neural-recording amplifier system using differentially regulated rejection ratio enhancement in 0.18 µm CMOS. In 2018 IEEE Int. Solid-State Circuits Conference (ISSCC) 472–474 (IEEE, 2018).
Harrison, R. R. & Charles, C. A low-power low-noise CMOS amplifier for neural recording applications. IEEE J. Solid-State Circ. 38, 958–965 (2003).
Zhao, Y., Shang, Z. & Lian, Y. A 2.55 NEF 76 dB CMRR DC-coupled fully differential difference amplifier based analog front end for wearable biomedical sensors. IEEE Trans. Biomed. Circ. Syst. 13, 918–926 (2019).
Lee, B. & Ghovanloo, M. An adaptive averaging low noise front-end for central and peripheral nerve recording. IEEE Transactions on Circuits and Systems II: Express Briefs 65, 839–843 (2017).
Muller, R. et al. A minimally invasive 64-channel wireless μecog implant. IEEE J. Solid-State Circ. 50, 344–359 (2014).
Kassiri, H. et al. 27.3 All-wireless 64-channel 0.013mm 2/ch closed-loop neurostimulator with rail-to-rail DC offset removal. In 2017 IEEE Int. Solid-State Circuits Conference (ISSCC) 452–453 (IEEE, 2017).
O’Leary, G. et al. A recursive-memory brain-state classifier with 32-channel track-and-zoom Δ2Σ ADCs and charge-balanced programmable waveform neurostimulators. In 2018 IEEE Int. Solid-State Circuits Conference (ISSCC) 296–298 (IEEE, 2018).
Jeon, H., Bang, J.-S., Jung, Y., Choi, I. & Je, M. A high DR, DC-coupled, time-based neural-recording IC with degeneration R-DAC for bidirectional neural interface. IEEE J. Solid-State Circ. 54, 2658–2670 (2019).
Leene, L. B. & Constandinou, T. G. A 0.006mm2 1.2µW analog-to-time converter for asynchronous bio-sensors. IEEE J. Solid-State Circ. 53, 2604–2613 (2018).
Haas, M. & Ortmanns, M. Efficient implementation and stability analysis of a HV-CMOS current/voltage mode stimulator. In 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS) https://doi.org/10.1109/BIOCAS.2018.8584804 (IEEE, 2018).
Butz, N., Taschwer, A., Manoli, Y. & Kuhl, M. 22.6 A 22V compliant 56µW active charge balancer enabling 100% charge compensation even in monophasic and 36% amplitude correction in biphasic neural stimulators. In 2016 IEEE Int. Solid-State Circuits Conference (ISSCC) 390–391 (IEEE, 2016).
Greenwald, E. et al. A CMOS current steering neurostimulation array with integrated DAC calibration and charge balancing. IEEE transactions on biomedical circuits and systems 11, 324–335 (2017).
Lee, H.-M., Kwon, K. Y., Li, W. & Ghovanloo, M. A power-efficient switched-capacitor stimulating system for electrical/optical deep brain stimulation. IEEE J. Solid-State Circ. 50, 360–374 (2014).
Sooksood, K., Stieglitz, T. & Ortmanns, M. An active approach for charge balancing in functional electrical stimulation. IEEE Transactions on Biomedical Circuits and Systems 4, 162–170 (2010).
Kwong, J. & Chandrakasan, A. P. An energy-efficient biomedical signal processing platform. IEEE J. Solid-State Circ. 46, 1742–1753 (2011).
Cong, P. et al. A 32-channel modular bi-directional neural interface system with embedded DSP for closed-loop operation. In European Solid State Circuits Conference (ESSCIRC) 99–102 (IEEE, 2014).
Alzuhair, A. & Marković, D. A 216 nW/channel DSP engine for triggering theta phase-locked brain stimulation. In 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS) https://doi.org/10.1109/BIOCAS.2017.8325189 (IEEE, 2017).
Rhew, H.-G. et al. A fully self-contained logarithmic closed-loop deep brain stimulation SoC with wireless telemetry and wireless power management. IEEE J. Solid-State Circ. 49, 2213–2227 (2014).
Altaf, M. A. B., Zhang, C. & Yoo, J. A 16-channel patient-specific seizure onset and termination detection SoC with impedance-adaptive transcranial electrical stimulator. IEEE J. Solid-State Circ. 50, 2728–2740 (2015).
Azin, M., Guggenmos, D. J., Barbay, S., Nudo, R. J. & Mohseni, P. A battery-powered activity-dependent intracortical microstimulation IC for brain-machine-brain interface. IEEE J. Solid-State Circ. 46, 731–745 (2011).
Iranmanesh, S. & Rodriguez-Villegas, E. A 950 nW analog-based data reduction chip for wearable EEG systems in epilepsy. IEEE J. Solid-State Circ. 52, 2362–2373 (2017).
Cheng, C.-H. et al. A fully integrated 16-channel closed-loop neural-prosthetic CMOS SoC with wireless power and bidirectional data telemetry for real-time efficient human epileptic seizure control. IEEE J. Solid-State Circ. 53, 3314–3326 (2018).
Pazhouhandeh, M. R. et al. 22.8 Adaptively clock-boosted auto-ranging responsive neurostimulator for emerging neuromodulation applications. In 2019 IEEE Int. Solid-State Circuits Conference (ISSCC), 374–376 (IEEE, 2019).
Imtiaz, S. A., Jiang, Z. & Rodriguez-Villegas, E. An ultralow power system on chip for automatic sleep staging. IEEE J. Solid-State Circ. 52, 822–833 (2017).
Chang, S.-Y. et al. An ultra-low-power dual-mode automatic sleep staging processor using neural-network-based decision tree. IEEE Trans. Circ. Syst. I 66, 3504–3516 (2019).
Chen, Y., Yao, E. & Basu, A. A 128-channel extreme learning machine-based neural decoder for brain machine interfaces. IEEE Trans. Biomed. Circ. Syst. 10, 679–692 (2016).
Harrison, R. R. The design of integrated circuits to observe brain activity. Proc. IEEE 96, 1203–1216 (2008).
Zhang, F., Mishra, A., Richardson, A. G. & Otis, B. A low-power ECoG/EEG processing IC with integrated multiband energy extractor. IEEE Trans. Circ. Syst. I 58, 2069–2082 (2011).
Karkare, V., Gibson, S. & Markovic, D. A 130-µW, 64-channel neural spike-sorting DSP chip. IEEE J. Solid-State Circ. 46, 1214–1222 (2011).
Wu, T. et al. A 16-channel nonparametric spike detection ASIC based on EC-PC decomposition. IEEE Trans. Biomed. Circ. Syst. 10, 3–17 (2016).
Chen, T.-C., Chen, K., Yang, Z., Cockerham, K. & Liu, W. A biomedical multiprocessor SoC for closed-loop neuroprosthetic applications. In IEEE Int. Solid-State Circuits Conference - Digest of Technical Papers 434–435 (IEEE, 2009).
Karkare, V., Gibson, S. & Marković, D. A 75-µW, 16-channel neural spike-sorting processor with unsupervised clustering. IEEE J. Solid-State Circ. 48, 2230–2238 (2013).
Do, A. T., Zeinolabedin, S. M. A., Jeon, D., Sylvester, D. & Kim, T. T.-H. An area-efficient 128-channel spike sorting processor for real-time neural recording with 0.175μW/channel in 65-nm CMOS. IEEE Trans. VLSI Syst. 27, 126–137 (2018).
Aprile, C. et al. Adaptive learning-based compressive sampling for low-power wireless implants. IEEE Trans. Circ. Syst. I 65, 3929–3941 (2018).
Ranjandish, R. & Schmid, A. A sub-µW/channel, 16-channel seizure detection and signal acquisition SoC based on multichannel compressive sensing. IEEE Trans. Circ. Syst. II 65, 1400–1404 (2018).
Liu, X. et al. A fully integrated wireless compressed sensing neural signal acquisition system for chronic recording and brain machine interface. IEEE Trans. Biomed. Circ. Syst. 10, 874–883 (2016).
Biederman, W. et al. A 4.78mm2 fully-integrated neuromodulation SoC combining 64 acquisition channels with digital compression and simultaneous dual stimulation. IEEE J. Solid-State Circ. 50, 1038–1047 (2015).
Kim, S.-J. et al. A sub-µW/Ch analog front-end for Δ-neural recording with spike-driven data compression. IEEE Trans. Biomed. Circ. Syst. https://doi.org/10.1109/TBCAS.2018.2880257 (2018).
Liu, X., Zhang, M., Richardson, A. G., Lucas, T. H. & Van der Spiegel, J. Design of a closed-loop, bidirectional brain machine interface system with energy efficient neural feature extraction and PID control. IEEE Trans. Biomed. Circ. Syst. 11, 729–742 (2017).
Venkatraman, S., Elkabany, K., Long, J. D., Yao, Y. & Carmena, J. M. A system for neural recording and closed-loop intracortical microstimulation in awake rodents. IEEE Trans. Biomed. Eng. 56, 15–22 (2009).
Mendrela, A. E. et al. A bidirectional neural interface circuit with active stimulation artifact cancellation and cross-channel common-mode noise suppression. IEEE J. Solid-State Circ. 51, 955–965 (2016).
Zhou, A., Johnson, B. C. & Muller, R. Toward true closed-loop neuromodulation: artifact-free recording during stimulation. Curr. Opin. Neurobiol. 50, 119–127 (2018).
Stanslaski, S. et al. Creating neural “co-processors” to explore treatments for neurological disorders. In IEEE Int. Solid-State Circuits Conference (ISSCC) 460–462 (IEEE, 2018).
Thomas, G. P. & Jobst, B. C. Critical review of the responsive neurostimulator system for epilepsy. Med. Dev. 8, 405 (2015).
Kohler, F. et al. Closed-loop interaction with the cerebral cortex: a review of wireless implant technology. Brain-Comp. Interf. 4, 146–154 (2017).
Xu, J. et al. A 160μW 8-channel active electrode system for EEG monitoring. IEEE Trans. Biomed. Circ. Syst. 5, 555–567 (2011).
Xu, J. et al. A 665µW silicon photomultiplier-based NIRS/EEG/EIT monitoring ASIC for wearable functional brain imaging. In IEEE Int. Solid-State Circuits Conference (ISSCC) 294–296 (IEEE, 2018).
Pfurtscheller, G. et al. The hybrid BCI. Front. Neurosci. 4, 30 (2010).
Schwarz, D. A. et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat. Methods 11, 670–676 (2014).
Angotzi, G. N., Boi, F., Zordan, S., Bonfanti, A. & Vato, A. A programmable closed-loop recording and stimulating wireless system for behaving small laboratory animals. Sci. Rep. 4, 5963 (2014).
Yin, M. et al. Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 84, 1170–1182 (2014).
Zhou, A. et al. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates. Nat. Biomed. Eng. 3, 15–26 (2019).
Serruya, M. D., Hatsopoulos, N. G., Paninski, L., Fellows, M. R. & Donoghue, J. P. Brain-machine interface: Instant neural control of a movement signal. Nature 416, 141–142 (2002).
Taylor, D. M., Tillery, S. I. H. & Schwartz, A. B. Direct cortical control of 3D neuroprosthetic devices. Science 296, 1829–1832 (2002).
Wolpaw, J. R. & McFarland, D. J. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc. Natl Acad. Sci. USA 101, 17849–17854 (2004).
Rebsamen, B. et al. A brain controlled wheelchair to navigate in familiar environments. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 590–598 (2010).
Chaudhary, U., Birbaumer, N. & Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 12, 513–525 (2016).
Tran, N. et al. A complete 256-electrode retinal prosthesis chip. IEEE J. Solid-State Circ. 49, 751–765 (2014).
Ramos-Murguialday, A. et al. Brain–machine interface in chronic stroke rehabilitation: a controlled study. Ann. Neurol. 74, 100–108 (2013).
Capogrosso, M. et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288 (2016).
Wagner, F. B. et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71 (2018).
Chortos, A., Liu, J. & Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 15, 937–950 (2016).
Tabot, G. A. et al. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc. Natl Acad. Sci. USA 18279–18284 (2013).
Lopez, C. M. et al. 22.7 A 966-electrode neural probe with 384 configurable channels in 0.13 µm SOI CMOS. In 2016 IEEE Int. Solid-State Circuits Conference (ISSCC) 392–393 (IEEE, 2016).
Herbawi, A. S., Kießner, L., Paul, O. & Rüther, P. High-density CMOS neural probe implementing a hierarchical addressing scheme for 1600 recording sites and 32 output channels. In 19th Int. Conf. Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 20–23 (IEEE, 2017).
De Dorigo, D. et al. A fully immersible deep-brain neural probe with modular architecture and a delta-sigma ADC integrated under each electrode for parallel readout of 144 recording sites. In IEEE Int. Solid-State Circuits Conference (ISSCC) 462–464 (IEEE, 2018).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci 14, 1599–1605 (2011).
Biederman, W. et al. A fully-integrated, miniaturized (0.125 mm2) 10.5 µW wireless neural sensor. IEEE J. Solid-State Circ. 48, 960–970 (2013).
Kim, C. et al. A 3 mm× 3 mm fully integrated wireless power receiver and neural interface system-on-chip. IEEE Trans. Biomed. Circ. Syst. 13, 1736–1746 (2019).
Yeon, P., Bakir, M. S. & Ghovanloo, M. Towards a 1.1 mm2 free-floating wireless implantable neural recording SoC. In 2018 IEEE Custom Integrated Circuits Conference (CICC) https://doi.org/10.1109/CICC.2018.8357048 (IEEE, 2018).
Khalifa, A. et al. The microbead: A 0.009mm3 implantable wireless neural stimulator. IEEE Trans. Biomed. Circ. Syst. 13, 971–985 (2019).
Leung, V. W. et al. A CMOS distributed sensor system for high-density wireless neural implants for brain-machine interfaces. In ESSCIRC 2018-IEEE 44th European Solid State Circuits Conference (ESSCIRC) 230–233 (IEEE, 2018).
Jia, Y. et al. A mm-sized free-floating wirelessly powered implantable optical stimulating system-on-a-chip. In IEEE Int. Solid-State Circuits Conference-(ISSCC) 468–470 (IEEE, 2018).
Ghanbari, M. M. et al. 17.5 A 0.8 mm 3 ultrasonic implantable wireless neural recording system with linear AM backscattering. In 2019 IEEE Int. Solid-State Circuits Conference (ISSCC) 284–286 (IEEE, 2019).
Charthad, J. et al. A mm-sized wireless implantable device for electrical stimulation of peripheral nerves. IEEE Trans. Biomed. Circ. Syst. 12, 257–270 (2018).
Neely, R. M., Piech, D. K., Santacruz, S. R., Maharbiz, M. M. & Carmena, J. M. Recent advances in neural dust: towards a neural interface platform. Curr. Opin. Neurobiol. 50, 64–71 (2018).
Leene, L. B. et al. Autonomous SoC for neural local field potential recording in mm-scale wireless implants. In 2018 IEEE Int. Symp. Circuits and Systems (ISCAS) https://doi.org/10.1109/ISCAS.2018.8351147 (IEEE, 2018).
Laiwalla, F. et al. A distributed wireless network of implantable sub-mm cortical microstimulators for brain-computer interfaces. In 2019 41st Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC) 6876–6879 (IEEE, 2019).
Naor, O., Krupa, S. & Shoham, S. Ultrasonic neuromodulation. J. Neural Eng. 13, 031003 (2016).
Gagnon-Turcotte, G., Ethier, C., De Köninck, Y. & Gosselin, B. A 13µm CMOS SoC for simultaneous multichannel optogenetics and electrophysiological brain recording. In 2018 IEEE Int. Solid-State Circuits Conference (ISSCC) 466–468 (IEEE, 2018).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Ding, H. et al. Microscale optoelectronic infrared-to-visible upconversion devices and their use as injectable light sources. Proc. Natl Acad. Sci. USA 115, 6632–6637 (2018).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2012).
Kim, T.-i et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Hodgkin, A. Evidence for electrical transmission in nerve. J. Physiol. 90, 183–210 (1937).
Acknowledgements
This work is supported in part by the Beijing Innovation Center for Future chip, in part by the Beijing National Research Center for Information Science and Technology, in part by the Natural Science Foundation of China through grant 61674095.
Author information
Authors and Affiliations
Contributions
M.Z. and J.V.d.S. conceived the work and suggested the outline of the paper. M.Z. and X.L. worked on the study of various neural interface designs. Z.T. and M.Z. carried out investigations and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, M., Tang, Z., Liu, X. et al. Electronic neural interfaces. Nat Electron 3, 191–200 (2020). https://doi.org/10.1038/s41928-020-0390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-020-0390-3
This article is cited by
-
In-vivo integration of soft neural probes through high-resolution printing of liquid electronics on the cranium
Nature Communications (2024)
-
Genetically targeted chemical assembly
Nature Reviews Bioengineering (2023)
-
Bibliometric analysis on Brain-computer interfaces in a 30-year period
Applied Intelligence (2023)
-
Chemically communicating with the brain through artificial neurons
Nature Electronics (2022)
-
Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics
Nature Communications (2022)