Abstract
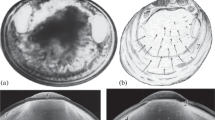
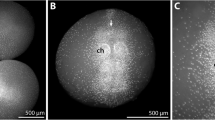
Members of family Cymatiidae have an unusually long planktonic larval life stage (veligers) which allows them to be carried within ocean currents and become distributed worldwide. However, little is known about these planktonic veligers and identification of the larval state of many Cymatiidae is challenging at best. Here, we describe the first high-quality scanning electron microscopy images of the developing veliger larvae of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: Cymatiidae). The developing shell of Monoplex veligers was captured by SEM, showing plates secreted to form the completed shell. The incubation time of the two species was recorded and found to be different; M. parthenopeus took 24 days to develop fully and hatch out of the egg capsules, whereas M. pilearis took over a month to leave the egg capsule. Using scanning electron microscopy and geometric morphometrics, the morphology of veliger larvae was compared. No significant differences were found between the shapes of the developing shell between the two species; however, it was found that M. pilearis was significantly larger than M. parthenopeus upon hatching. Although statistical analysis did not find morphological differences, this study concludes biological differences do exist between these two closely related species of Monoplex.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article and supplementary material. All images generated during this study are available from the authors upon reasonable request.
Abbreviations
- PCA:
-
Principal component analysis
- SEM:
-
Scanning electron microscopy
References
Abalde, S. L., Fuentes, J., & Gonzalez-Fernandez, A. (2003). Identification of Mytilus galloprovincialis larvae from the Galician rias by mouse monoclonal antibodies. Aquaculture, 219, 545–559.
Andrade-Villagran, P. V., Baria, K. S., Montory, J. A., Pechenik, J. A., & Chaparro, O. R. (2018). The influence of encapsulated embryos on the timing of hatching in the brooding gastropod Crepipatella dilatata. Journal of Sea Research, 131, 69–78.
Andrade-Villagrán, P. V., Mardones-Toledo, D. A., Paredes-Molina, F. J., Salas-Yanquin, L. P., Pechenik, J. A., Matthews-Cascon, H., & Chaparro, O. R. (2018). Possible mechanisms of hatching from egg capsules in the gastropods Crepipatella dilatata and Crepipatella peruviana, species with different modes of early development. The Biological Bulletin, 234, 69–84.
Avaca, M. S., Narvarte, M., Martin, P., & van der Molen, S. (2013). Shell shape variation in the Nassariid Buccinanops globulosus in northern Patagonia. Helgoland Marine Research, 67, 567–577.
Beesley, P.L., Ross, G.J.B., Wells, A. (1998). Mollusca: the southern synthesis. Fauna of Australia Part A, Volume 5.
Beu, A. G., & Knudsen, J. (1987). Taxonomy of gastropods of the families Ranellidae (= Cymatiidae) and Bursidae. Part 3. A review of the Trifidribbed species of Cymatium (Turritriton). Journal of the Royal Society of New Zealand, 73–91.
Board, W.E. (2018). World register of marine species database.
Chaparro, O. R., Cubillos, V. M., Montiel, Y. A., Paschke, K. A., & Pechenik, J. A. (2008). Embryonic encapsulation and maternal incubation: requirements for survival of the early stages of the estuarine gastropod Crepipatella delatata. Journal of Experimental Marine Biology and Ecology, 365, 38–45.
Chaparro, O. R., Cubillos, V. M., Montory, J. A., Navarro, J. M., & Andrade-Villagrán, P. V. (2019). Reproductive biology of the encapsulating, brooding gastropod Crepipatella dilatata Lamarck (Gastropoda, Calyptraeidae). PLoS One, 14, e0220051.
Conde-Padin, P., Grahame, J. W., & Rolan-Alvarez, E. (2007). Detecting shape differences in species of the Littorina saxatilis complex by morphometric analysis. Journal of Molluscan Studies, 73, 147–154.
Doyle, D., Gammell, M. P., & Nash, R. (2018). Morphometric methods for the analysis and classification of gastropods: a comparison using Littorina littorea. Journal of Molluscan Studies, 1–8.
Gordillo, S., Marquez, F., Cardenas, J., & Zubimendi, M. (2011). Shell variability in Tawera gayi (Veneridae) from southern South America: a morphometric approach based on contour analysis. Journal of the Marine Biological Association of the United Kingdom, 91, 815–822.
Haines, A. J., & Crampton, J. S. (2000). Improvements to the method of Fourier shape analysis as applied in morphometric students. Paleontology, 43, 765–783.
Hendriks, I., Luca, A., van Duren, P., & Herman, M. J. (2005). Image analysis techniques: a tool for the identification of bivalve larvae? Journal of Sea Research, 54, 151–162.
Hickman, C. (1999). Adaptive function of gastropod larval shell features. Invertebrate Biology, 118, 346–356.
Hickman, C. S. (2001). Evolution and development of gastropod larval shell morphology: experimental evidence for mechanical defense and repair. Evolution & Development, 3, 18–23.
Jablonski, D., & Lutz, R. (1983). Larval ecology of marine benthic invertebrates: paleobiological implications. Biological Reviews, 58, 21–89.
Kano, Y., & Kase, T. (2008). Diversity and distributions of the submarine-cave Neritiliidae in the Indo-Pacific (Gastropoda: Neritimorpha). Organisms, Diversity and Evolution, 8, 22–43.
Koca, A., Moradi, M., Deliklitas, O., Ucan, A., & Kandemir, I. (2018). Discrimination of dwarf honey bee (Apis florea, Fabricius 1876) populations in Iran using elliptic Fourier wing cell shape analysis. Evolution, phylogeny, and biogeography, 57, 195–202.
Laxton, J. H. (1969). Reproduction in some New Zealand Cymatiidae (Gastropoda: Prosobranchia). Zoological Journal of the Linnean Society, 48, 237–253.
Marquez, F., Primost, M. A., & Bigatti, G. (2017). Shell shape as a biomarker of marine pollution historic increase. Marine Pollution Bulletin, 114, 816–820.
Montory, J. A., Chaparro, O. R., Pechenik, J. A., Diederich, C. M., & Cubillos, V. M. (2014). Impact of short-term salinity stress on larval development of the marine gastropod Crepipatella fecunda (Calyptraeidae). Journal of Experimental Marine Biology and Ecology, 458, 39–45.
Muthiah, P., & Sampath, K. (2000a). Early development of Cymatium (Monoplex) pilearis and C. (Linatella) cutaceum (Ranellidae: Gastropoda: Mollusca) in the laboratory. Indian Journal of Fisheries, 47, 201–207.
Muthiah, P., & Sampath, K. (2000b). Spawn and fecundity of Cymatium (Monoplex) pilearis and Cymatium (Linatella) cingulatum (Gastropoda: Ranellidae). Journal of Molluscan Studies, 66, 293–300.
Pechenik, J., Scheltema, R. S., & Eyster, L. S. (1984). Growth stasis and limited shell calcification in larvae of Cymatium parthenopeum during trans-Atlantic transport. Science, 224, 1097–1099.
Penchaszadeh, P., Teso, V., & Pastorino, G. (2017). Spawn in two deep-sea volute gastropods (Neogastropoda: Volutidae) from southwestern Atlantic waters. Deep-Sea Research Part 1, 130, 55–62.
Pennington, J. T., & Chia, F. S. (1985). Gastropod torsion: a test of Garstang’s hypothesis. The Biological Bulletin, 169, 391–396.
Robertson, R. (1971). Scanning electron microscopy of planktonic larval marine gastropod shells. The Veliger, 14, 2–12.
Rohlf F (2006a) TPSDIG2. Department of Ecology and Evolution, State University of New York at Stony Brook, New York.
Rohlf F (2006b) TPSUtil. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, pp file utility program.
Rohlf F (2007a) TPSRegr. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, pp shape regression.
Rohlf F (2007b) TPSRelw. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, pp relative warp analysis.
Rohlf, F., & Marcus, L. F. (1993). A revolution in morphometrics. Trends in Ecology & Evolution, 8, 129–132.
Rohlf, F., & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Sang, S., Friend, D. S., Allmon, W. D., & Anderson, B. M. (2019). Protoconch enlargement in Western Atlantic turritelline gastropod species following the closure of the Central American Seaway. Ecology and Evolution, 9, 5309–5323.
Scheltema, R. (1966). Evidence for trans-Atlantic transport of gastropod larvae belonging to the genus Cymatium. Deep Sea Research, 13, 83–95.
Solsona, M., & Martinell, J. (1999). Protoconch as a taxonomic tool in Gastropoda systematics. Application in the Pleiocene Mediterranean Naticidae. Geobios, 32, 409–419.
Sparagano, O. A., Purdom, I., Priest, F. G., & Kingston, P. F. (2002). Molecular characterization of the bivalves Mya arenaria, Mya truncata and Hiatella arctica. Journal of Molluscan Studies, 68, 190–191.
Strathmann, M. F., & Strathmann, R. R. (2007). An extraordinarily long larval duration of 4.5 years from hatching to metamorphosis for teleplanic veligers of Fusitriton oregonensis. The Biological Bulletin, 213, 152–159.
Claxton, W., & Boulding, E. (1998). A new molecular technique for identifying field collections of zebra mussel (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) veliger larvae applied to eastern Lake Erie, Lake Ontario, and Lake Simcoe. Canadian Journal of Zoology, 76, 194–198.
Wang, S., Bao, Z., Zhang, L., Li, N., Zhan, A., Guo, W., Wang, X., & Hu, J. (2006). A new strategy for species identification of planktonic larvae: PCR-RFLP analysis of the internal transcribed spacer region of ribosomal DNA detected by agarose gel electrophoresis or DHPLC. Journal of Plankton Research, 28, 375.
Wisherkerman, A., & Hamilton, P. (2018). Shape outline extraction software (DiaOutline) for elliptic Fourier analysis application in morphometric studies. Applications in Plant Sciences, 6.
Acknowledgements
We thank Kathryn Green and Nicolas Condon from the Center for Microscopy and Microanalysis at the University of Queensland, St. Lucia; the UQ Biological Resources Department personnel for care of the tritons in aquaria, Mr. Greg Knight and the employees of his oyster lease, the Clout family of Kooringal Oysters, and everyone at Moreton Bay Rock Oyster Company for donating specimens of M. parthenopeus. Thanks also to Dr. David Anning for assisting in collecting M. pilearis and Dr. John Healy, Curator of Molluscs at the Queensland Museum, for his expertise and assistance in specimen identification.
Funding
This research was funded by Australian Research Council (ARC) Discovery Project DP150103990 awarded to Q.K. and D.J.C. A.H.T. was supported by a University of Queensland Research Training Tuition Offset Scholarship and a University of Queensland Training Program Living Allowance Scholarship, C.I.S. was supported by an ARC Future Fellowship (FT160100055) and an Institute for Molecular Bioscience (IMB) Industry Fellowship, and D.J.C. was supported by an ARC Australian Laureate Fellowship (FL150100146).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. See University of Queensland Animal Ethics Committee definition of animal research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1255 kb)
Rights and permissions
About this article
Cite this article
Turner, A.H., Kaas, Q., Craik, D.J. et al. Early development of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: Cymatiidae): biology and morphology. Org Divers Evol 20, 51–62 (2020). https://doi.org/10.1007/s13127-020-00432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-020-00432-5