Abstract
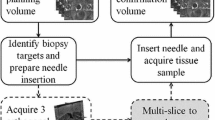
Transrectal ultrasound (TRUS) guided needle biopsy is currently the clinical routine for diagnosis of prostate cancer. Due to the blurry TRUS imaging, magnetic resonance imaging (MRI) of prostate is usually taken prior to the biopsy procedure to assist needle target planning. In this paper, a novel geometric transformation method, which is based on the mechanics of materials, is proposed to facilitate the deformable registration of lesion sites from MRI to ultrasound imaging. In the method an enlarged shell, scaled from the geometry of prostate constructed from ultrasound imaging, is used to squeeze the prostate constructed from MRI imaging. The prostate can automatically rotate and position itself towards the target geometry, and deforms to match the target throughout the entire volumes. Transformations of 3D volumes as well as 2D sections of prostate are carried out to evaluate the transformation performance with respect to different material properties and segmentation errors. Some practical issues on applying the method to clinical biopsy operations are discussed. We show that this FEM based transformation method is promising in deformable registration and could be used in other organ image registrations.












Similar content being viewed by others
References
Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y (2013) Systematic review of complications of prostate biopsy. Eur Urol 64:876–892
Onik G, Barzell W (2008) Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 26:506–510
Komai Y, Numao N, Yoshida S, Matsuoka Y, Nakanishi Y, Ishii C, Koga F, Saito K, Masuda H, Fujii Y, Kawakami S, Kihara K (2013) High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol 190:867–873
Kuru TH, Roethke MC, Seidenader J, Simpfendörfer T, Boxler S, Alammar K, Rieker P, Popeneciu VI, Roth W, Pahernik S, Schlemmer HP, Hohenfellner M, Hadaschik BA (2013) Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol 190:1380–1386
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, Hoang AN, Walton-Diaz A, Shuch B, Weintraub M, Kruecker J, Amalou H, Turkbey B, Merino MJ, Choyke PL, Wood BJ, Pinto PA (2013) Magnetic resonance imaging/ultrasound–fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 64:713–719
Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, Turkbey B, Gupta GN, Kruecker J, Linehan WM, Choyke PL, Wood BJ, Pinto PA (2012) Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 188:2152–2157
Flard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, Long JA (2013) Targeted MRI-guided prostate biopsies for the detection of prostate cancer: initial clinical experience with real-time 3-Dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology 81(6):1372–1378
Karnik VV, Fenster A, Bax J, Cool DW, Gardi L, Gyacskov I, Romagnoli C, Ward AD (2010) Assessment of image registration accuracy in three-dimensional transrectal ultrasound guided prostate biopsy. Med Phys 37:802–813
Ou Y, Sotiras A, Paragios N, Davatzikos C (2011) DRAMMS: deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal 15:622–639
Yang X, Akbari H, Halig L, Fei B (2011) 3D non-rigid registration using surface and local salient features for transrectal ultrasound image-guided prostate biopsy. Proc SPIE 7964:79642V-1–79642V-8
Baumann M, Mozer P, Daanen V, Troccaz J (2012) Prostate biopsy tracking with deformation estimation. Med Image Anal 16:562–576
Mitra J, Kato Z, Marti R, Oliver A, Lladó X, Sidibé D, Ghose S, Vilanova JC, Comet J, Meriaudeau F (2012) A spline-based non-linear diffeomorphism for multimodal prostate registration. Med Image Anal 16:1259–1279
Pursley J, Risholm P, Fedorov A, Tuncali K, Fennessy FM, Wells WM, Tempany CM, Cormack RA (2012) A Bayesian nonrigid registration method to enhance intraoperative target definition in image-guided prostate procedures through uncertainty characterization. Med Phys 39:6858–6867
Boubaker MB, Haboussi M, Ganghoffer JF, Aletti P (2009) Finite element simulation of interactions between pelvic organs: predictive model of the prostate motion in the context of radiotherapy. J Biomech 42:1862–1868
Chai X, van Herk M, van de Kamer JB, Hulshof MC, Remeijer P, Lotz HT, Bel A (2011) Finite element based bladder modeling for image-guided radiotherapy of bladder cancer. Med Phys 38:142–150
Alterovitz R, Goldberg K, Pouliot J, Hsu IC, Kim Y, Noworolski SM, Kurhanewicz J (2006) Registration of MR prostate images with biomechanical modeling and nonlinear parameter estimation. Med Phys 33:446–454
Hensel JM, Ménard C, Chung PW, Milosevic MF, Kirilova A, Moseley JL, Haider MA, Brock KK (2007) Development of multiorgan finite element-based prostate deformation model enabling registration of endorectal coil magnetic resonance imaging for radiotherapy planning. Int J Radiat Oncol Biol Phys 68(5):1522–1528
Hu Y, van den Boom R, Carter T, Taylor Z, Hawkes D, Ahmed HU, Emberton M, Allen C, Barratt D (2010) A comparison of the accuracy of statistical models of prostate motion trained using data from biomechanical simulations. Prog Biophys Mol Biol 103:262–272
Hu Y, Ahmed HU, Taylor Z, Allen C, Emberton M, Hawkes D, Barratt D (2012) MR to ultrasound registration for image-guided prostate interventions. Med Image Anal 16:687–703
Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M (2014) Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol 65:628–634
Wang Y, Cheng JZ, Ni D, Lin M, Qin J, Luo X, Xu M, Xie X, Heng PA (2016) Towards personalized statistical deformable model and hybrid point matching for robust MR-TRUS registration. IEEE Trans Med Imaging 35:589–604
Misra S, Macura KJ, Ramesh KT, Okamura AM (2009) The importance of organ geometry and boundary constraints for planning of medical interventions. Med Eng Phys 31:195–206
Carson WC, Gerling GJ, Krupski TL, Kowalik CG, Harper JC, Moskaluk CA (2011) Material characterization of ex vivo prostate tissue via spherical indentation in the clinic. Med Eng Phys 33:302–309
Rubod C, Brieu M, Cosson M, Rivaux G, Clay JC, de Landsheere L, Gabriel B (2012) Biomechanical properties of human pelvic organs. Urology 79(4):968.e17–968.e22
Cool D, Downey D, Izawa J, Chin J, Fenster A (2006) 3D prostate model formation from non-parallel 2D ultrasound biopsy images. Med Image Anal 10:875–887
Martin S, Troccaz J, Daanenc V (2010) Automated segmentation of the prostate in 3D MR images using a probabilistic atlas and a spatially constrained deformable model. Med Phys 37:1579–1590
Mahdavi SS, Chng N, Spadinger I, Morris WJ, Salcudean SE (2011) Semi-automatic segmentation for prostate interventions. Med Image Anal 15:226–237
Khalvati F, Salmanpour A, Rahnamayan S, Rodrigues G, Tizhoosh HR (2013) Inter-slice bidirectional registration-based segmentation of the prostate gland in MR and CT image sequences. Med Phys 40:123503
Acknowledgements
The authors thank A*STAR JCO Program—(Grant Number—1231BFG044) for funding this research and thank the Institute of High Performance Computing and Singapore Bioimaging Consortium for the use the computational resources to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, F., Liu, J. Prostate deformable registration through geometric transformation by finite element method. Meccanica 55, 669–680 (2020). https://doi.org/10.1007/s11012-019-01105-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11012-019-01105-0