Abstract
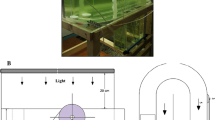
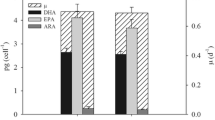
The microalga Dunaliella salina was studied at the main stages of transition from laboratory to pilot-scale cultivation: strain selection, nutrient medium selection, estimation of influence of physicochemical factors on accumulation of carotenoids and evaluation of the selected strain growing technology at pilot conditions. Dunaliella salina strain IBSS-2 was recognized as promising for commercial cultivation due to the combination of high-production characteristics, environmental stress resistance, and relative easiness of transition to the carotenogenesis stage. The influence of stress factors on the D. salina culture productivity in two nutrient media was estimated. It was shown that light effect combined with nutrients deficiency is the key factor for β-carotene accumulation. The influence of increased irradiance caused the increase of carotenoid content in D. salina cells up to 8%, and increasing irradiance and salinity resulted in carotenoid productivity going up 1.5 times. Testing of D. salina pilot cultivation system demonstrated that productivity at the first cultivation stage was about 6 g m−2 day−1 in both batch and semicontinuous mode. During pilot D. salina cultivation in Crimea, the culture transition to the carotenogenesis stage was achieved both in summer and autumn. The concentration of carotenoids in the ponds was 200 and 600 mg m−2 with a carotenoid/chlorophyll a (Car/Chl a) ratio of 7 and 4.5 in summer and autumn, respectively. The possibility to use natural population of D. salina cells from salterns as inoculum and brine as a nutrient medium base was demonstrated. The study results suggest that the proposed approach can be recommended for D. salina commercial cultivation.







Similar content being viewed by others
References
Abdulagatov IM, Alkhasov AB, Dogeev GD, Tumalaev NR, Aliev RM, Badavov GB, Aliev A, Salikhova AS (2018) Technological application of microalgae in power industry and environmental protection. South of Russia: Ecology, Development 1:166–183 (In Russian)
Béchet Q, Moussion P, Bernard O (2017) Calibration of a productivity model for the microalgae Dunaliella salina accounting for light and temperature. Algal Res 21:156–160
Ben-Amotz A (1987) Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben Amotz and Avron (Volvocales, Chlorophyta). J Plant Physiol 131:479–487
Ben-Amotz A (1995) New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol 7:65–68
Ben-Amotz A (2009) Bioactive compounds: glycerol production, carotenoid production, fatty acids production. In: Ben-Amotz A, Polle JEW, Subba Rao DW (eds) The alga Dunaliella, biodiversity, physiology, genomics and biotechnology. CRC Press, Boca Raton, pp 189–207
Ben-Amotz A, Katz A, Avron M (1982) Accumulation of β-carotene in halotolerant alge: purification and characterization of β-carotene-rich globules from Dunaliella bardawil (Chlorophyceae). J Phycol 4:529–537
Bonnefond H, Moelants N, Talec A, Bernard O, Sciandra A (2016) Concomitant effects of light and temperature diel variations on the growth rate and lipid production of Dunaliella salina. Algal Res 14:72–78
Borovkov AB, Gudvilovich IN (2012) Growth and biochemical parametrs of Dunaliella salina in the conditions of semicontinuous culture. Biotechnologia Acta 5:105–111 (In Russian)
Borovkov AB, Gudvilovich IN (2013) Growth and biochemical indices of Dunaliella salina under conditions of batch culture. Hydrobiol J 49:75–84
Borovkov AB, Gudvilovich IN (2015) Intensive cultivation of Dunaliella salina for production of biomass with elevated β-carotene content. Communication 1. Effect of cultivation factors Hydrobiol J 51:69–76
Borovkov AB, Gudvilovich IN, Avsiyan AL, Memetshaeva OA, Lelekov AS, Novikova TM (2020) Production characteristics of Dunaliella salina at two-phase pilot cultivation (Crimea). Turk J Fish Aquat Sc 20:401–408
Borowitzka MA (1990) The mass culture of Dunaliella salina. In: Technical resource papers regional workshop on the culture and utilization of Seaweads (Vol. 2). http://www.fao.org/3/ab728e/AB728E06.htm accessed 16.02.2020
Borowitzka MA (2013) Dunaliella: biology, production, and markets. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. John Wiley & Sons, Ltd, Chichester, pp 359–368
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Borowitzka LJ, Borowitzka MA (1990) Commercial production of β-carotene by Dunaliella salina in open ponds. Bull Mar Sci 47:244–252
Borowitzka MA, Siva CJ (2007) The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J Appl Phycol 19:567–590
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52:407–418
Borowitzka LJ, Borowitzka MA, Moulton TP (1984) The mass culture of Dunaliella salina for fine chemicals: from laboratory to pilot plant. Hydrobiologia 116/117:115–121
Borowitzka MA, Borowitzka LJ, Kessly D (1990) Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J Appl Phycol 2:111–119
Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotech 74:1163–1174
García-González M, Moreno J, Cañavate JP, Anguis V, Prieto A, Manzano C, Florencio FJ, Guerrero MG (2003) Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. J Appl Phycol 15:177–184
Gevorgiz RG, Alisievich AV, Shmatok MG (2005) Estimation of biomass Spirulina platensis (Nordst.) Geitl with use of optical density of culture. Ecologiya Morya 70:96–106 (In Russian)
Gudvilovich IN (2010) Dunaliella salina of salt reservoirs at the western part of Crimea. Geopolitika i Ekogeodinamika Regionov 1:44–48 (In Russian)
Ishika T, Bahri PA, Laird DW, Moheimani NR (2018) The effect of gradual increase in salinity on the biomass productivity and biochemical composition of several marine, halotolerant, and halophilic microalgae. J Appl Phycol 30:1453–1464
Jin E, Polle JE (2009) Carotenoid biosynthesis in Dunaliella (Chlorophyta). In: Ben-Amotz A, Polle JEW, Subba Rao DW (eds) The alga Dunaliella, biodiversity, physiology, genomics and biotechnology. CRC Press, Boca Raton, pp 159–183
Lamers PP, Van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648
Lelekov AS, Trenkenshu RP (2007) The simplest models of microalgae growth. 4. Exponential and linear growth phases. Ecologiya Morya 74:47–49 (In Russian)
Lelekov AS, Borovkov AB, Novikova TM, Gudvilovich IN, Avsiyan AL, Memetshayeva OA (2019) Modeling the dynamics of pigment content in cells of Dunaliella salina Teod. unicellular alga at the stage of carotenogenesis. Math Biol Bioinf 14:279–289 (In Russian)
Lv H, Cui X, Tan Z, Jia S (2017) Analysis of metabolic responses of Dunaliella salina to phosphorus deprivation. J Appl Phycol 29:1251–1260
Massyuk NP (1973) Morphology, taxonomy, ecology, geographical distribution of the genus Dunaliella Teod. and perspective for its practical use. Nauk. Dumka Publ., Kiev. (In Russian)
Moulton TP, Borowitzka LJ, Vincent DJ (1987) The mass culture of Dunaliella salina for ß-carotene: from pilot plant to production plant. Hydrobiologia 151/152:99–105
Peel MC, Finlayson B, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11:1633–1644
Polle JE, Tran D, Ben-Amotz A (2009) History, distribution, and habitats of algae of the genus Dunaliella Teodoresco (Chlorophyceae). In: Ben-Amotz A, Polle JEW, Subba Rao DW (eds) The alga Dunaliella, biodiversity, physiology, genomics and biotechnology. CRC Press, Boca Raton, pp 1–14
Posudin YI, Massjuk NP, Lilitskaya GG (2010) Photomovement of Dunaliella Teod. Vieweg+Teubner Verlag, Wiesbaden
Prieto A, Cañavatea JP, García-González M (2011) Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol 151:180–185
Shaish A, Avron M, Ben-Amotz A (1990) Effect of ingibitors on the formation of stereoisomers in the biosynthesis of β-carotene in Dunaliella bardawil. Plant Cell Physiol 31:689–696
Tafreshi AH, Shariati M (2006) Pilot culture of three strains of Dunaliella salina for β-carotene production in open ponds in the central region of Iran. World J Microb Biotech 22:1003–1006
Terentieva NV, Minyuk GS, Drobetskaya IV, Chubchikova IN (2008) Features of secondary carotenogenesis in vegetative cells of Haematococcus pluvialis Flotow (Chlorophyceae) under different conditions of mineral supply. Morskoj Ehkologicheskij Zhurnal 7:66–74 (In Russian)
Trenkenshu RP, Belyanin VN (1979) The effect of mineral nutrients on algae Platymonas viridis Rouch. productivity. Russ J Mar Biol 51:41–46 (In Russian)
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Phys 144:307–313
Wu Z, Dejtisakdi W, Kermanee P, Ma C, Arirob W, Sathasivam R, Juntawong N (2017) Outdoor cultivation of Dunaliella salina KU 11 using brine and saline lake water with raceway ponds in northeastern Thailand. Biotechnol Appl Biochem 64:938–943
Xu Y, Ibrahim IM, Harvey PJ (2016) The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (Chlorophyta) CCAP 19/30. Plant Physiol Biochem 106:305–315
Zhu C, Zhai X, Jia J, Wang J, Han D, Li Y, Yajie T, Chi Z (2018) Seawater desalination concentrate for cultivation of Dunaliella salina with floating photobioreactor to produce β-carotene. Algal Res 35:319–324
Funding
This work was supported by the Russian Foundation for Basic Research [grant number 18-44-920009] and A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS [state project № АААА-А18-118021350003-6].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borovkov, A.B., Gudvilovich, I.N. & Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: from strain selection to open ponds. J Appl Phycol 32, 1545–1558 (2020). https://doi.org/10.1007/s10811-020-02104-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02104-5