Abstract
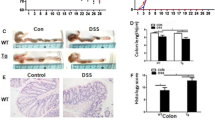
Intestinal inflammatory reactions and resulting tissue injuries are two major aspects of inflammatory bowel disease (IBD). The regulatory factors involved in the pathogenesis of IBD remain unclear. Recent studies showed that musculin (MSC) as a transcription suppressor participates in the regulation of certain immune functions. The purpose of this study was to determine the impact of MSC deficiency on colonic injury and inflammatory reaction under IBD, where wild-type (WT, +/+) and MSC-knockout (MSCKO, MSC−/−) mice were induced for disease by dextran sulfate sodium (DSS) in drinking water. Immunohistochemistry hematoxylin-eosin (H&E) staining, enzyme-linked immunosorbent assay (ELISA), and quantitative real-time polymerase chain reaction (qRT-PCR) were used to analyze the matching samples from groups of different genotypes. The colonic epithelial injury in the MSC−/− IBD group was much severer than that in the +/+ IBD group, concurrent with higher IL-22 levels from the supernatant of ex vivo cultured colon tissues in the MSC−/− IBD group than those in the +/+ IBD group. The mRNA levels of IL-22 in mesenteric lymph nodes (MLN) also manifested similar tendency. MSC deficiency may enhance the inflammatory reactions in the gut via excessive secretion of IL-22, leading to aggravated colonic epithelial injury under IBD.




Similar content being viewed by others
References
Kaser, A., S. Zeissig, and R.S. Blumberg. 2010. Inflammatory bowel disease. Annual Review of Immunology 28: 573–621.
Khor, B., A. Gardet, and R.J. Xavier. 2011. Genetics and pathogenesis of inflammatory bowel disease. Nature 474 (7351): 307–317.
Lu, J., R. Webb, J.A. Richardson, and E.N. Olson. 1999. MyoR: A muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD. Proceedings of the National Academy of Sciences of the United States of America 96 (2): 552–557.
Massari, M.E., R.R. Rivera, J.R. Voland, M.W. Quong, T.M. Breit, J.J. van Dongen, O. de Smit, and C. Murre. 1998. Characterization of ABF-1, a novel basic helix-loop-helix transcription factor expressed in activated B lymphocyte. Molecular and Cellular Biology 18 (6): 3130–3139.
Lu, J.R., R. Bassel-Duby, A. Hawkins, P. Chang, R. Valdez, H. Wu, L. Gan, J.M. Shelton, J.A. Richardson, and E.N. Olson. 2002. Control of facial muscle development by MyoR and capsulin. Science 298: 2378–2381.
Yu, L., J. Mikloucich, N. Sanqster, A. Perez, and P.J. McCormick. 2003. MyoR is expressed in nonmyogenic cells and can inhibit their differentiation. Experimental Cell Research 289 (1): 162–173.
Debuisson, D., N. Mari, S. Denanqlaire, O. Leo, and F. Andris. 2013. Myor/ABF-1 mrna expression marks follicular helper T cells but is dispensable for Tfh cell differentiation and function in vivo. PLoS One 8 (12): e84415.
Wu, C., Z. Chen, V. Dardalhon, S. Xiao, T. Thalhamer, M. Liao, A. Madi, R.F. Franca, T. Han, M. Oukka, and V. Kuchroo. 2017. The transcription factor musculin promotes the unidirectional development of peripheral Treg cells by suppressing the TH2 transcriptional program. Nature Immunology 18 (3): 344–353.
Santarlasci, V., A. Mazzoni, M. Capone, M.C. Rossi, L. Maggi, G. Montaini, B. Rossettini, R. Cimaz, M. Ramazzotti, G. Barra, R. De Palma, E. Maggi, F. Liotta, L. Cosmi, S. Romagnani, and F. Annunziato. 2017. Musculin inhibits human T-helper 17 cell response to interleukin 2 by controlling STAT5B activity. European Journal of Immunology 47 (9): 1427–1442.
Yu, J., W.Q. Tang, X. Yang, W. Ma, J. Yan, and H.P. Liang. 2018. Establisment of a transgenic mouse model with musculin knockout and Foxp3-GFP knockin and role of musculin in severe trauma-induced Treg-Th17 imblance. Journal of Third Military Medical University 40 (16): 1435–1443.
Silva, F.A., B.L. Rodrigues, M.L. Ayrizono, and R.F. Leal. 2016. The immunological basis of inflammatory bowel disease. Gastroenterology Research and Practice 2097274.
Kaplan, G.G. 2015. The global burden of IBD: From 2015 to 2025. Nature Reviews. Gastroenterology & Hepatology 12: 720–727.
Loftus, E.V. 2004. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterol 126 (6): 1504–1517.
Shindo, Y., A.G. Fuchs, C.G. Davis, T. Eitas, J. Unsinger, C.D. Burnham, J.M. Green, M. Morre, G.V. Bochicchio, and R.S. Hotchkiss. 2017. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. Journal of Leukocyte Biology 101 (2): 543–554.
Guo, X., J. Qiu, T. Tu, X. Yang, L. Deng, R.A. Anders, L. Zhou, and Y.X. Fu. 2014. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40 (1): 25–39.
Jinnohara, T., T. Kanaya, K. Hase, S. Sakakibara, T. Kato, N. Tachibana, T. Sasaki, Y. Hashimoto, T. Sato, H. Watarai, J. Kunisawa, N. Shibata, I.R. Williams, H. Kiyono, and H. O-hno. 2017. IL-22BP dictates characteristics of Peyer’s patch follicle-associated epithelium for antigen uptake. The Journal of Experimental Medicine 214 (6): 1607–1618.
Sabat, R., W. Ouyang, and K. Wolk. 2014. Therapeutic opportunities of the IL-22-IL-22R1 system. Nature Reviews. Drug Discovery 13 (1): 21–38.
Perusina Lanfranca, M., Y. Lin, J. Fang, W. Zou, and T. Frankel. 2016. Biological and pathological activities of interleukin-22. Journal of Molecular Medicine (Berlin, Germany) 94 (5): 523–534.
Brandtzaeg, P. 2002. The secretory immunoglobulin system: Regulation and biological significance. Focusing on human mammary glands. Advances in Experimental Medicine and Biology 503 (4): 1–16.
Bostick, J.W., and L. Zhou. 2016. Innate lymphoid cells in intestinal immunity and inflammation. Cellular and Molecular Life Sciences 73 (2): 237–252.
Peters, C.P., J.M. Mjösberg, J.H. Bernink, and H. Spits. 2016. Innate lymphoid cells in inflammatory bowel diseases. Immunology Letters 172: 124–131.
Forkel, M., and J. Mjösberg. 2016. Dysregulation of group 3 innate lymphoid cells in the pathogenesis of inflammatory bowel disease. Current Allergy and Asthma Reports 16 (10): 73.
Geremia, A., and C.V. Arancibia-Cárcamo. 2017. Innate lymphoid cells in intestinal inflammation. Frontiers in Immunology 8: 1296.
Klose, C.S., and D. Artis. 2016. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nature Immunology 17 (7): 765–774.
Zhao, P., and E.P. Hoffman. 2006. Musculin isoforms and repression of MyoD in muscle regeneration. Biochemical and Biophysical Research Communications 342: 835–842.
Moncaut, N., J.W. Cross, C. Siligan, A. Keith, K. Taylor, P.W. Rigby, and J.J. Carvajal. 2012. Musculin and TCF21 coordinate the maintenance of myogenic regulatory factor expression levels during mouse craniofacial development. Development 139 (5): 958–967.
Mathas, S., M. Janz, F. Hummel, M. Hummel, B. Wollert-Wulf, S. Lusatis, I. Anagnostopoulos, A. Lietz, M. Sigvardsson, F. Jundt, K. Jöhrens, K. Bommert, H. Stein, and B. Dörken. 2006. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nature Immunology 7 (2): 207–215.
Chiu, Y.K., I.Y. Lin, S.T. Su, K.H. Wang, S.Y. Yang, D.Y. Tsai, Y.T. Hsieh, and K.I. Lin. 2014. Transcription factor ABF-1 suppresses plasma cell differentiation but facilitates memory B cell formation. Journal of Immunology 193 (5): 2207–2217.
Acknowledgments
The authors would like to thank X Yan for editing the manuscript in English grammar and spelling. In addition, we also do appreciate Y S, W M, and X C for their kind statistical assistance and technological support.
Funding
This study was supported by the General Program of National Natural Science Foundation of China (81471863) (J Yan), the Special Project of Basic Science and Frontier Technology Research of Chongqing (cstc2016jcyjA0048) (J Yu), Natural Science Foundation of Chongqing (cstc 2018jcyjAX0258) (J Yan), and the Project for Enhancing Scientific and Technological Innovation Capabilities of Army Military Medical University (2019XYY22) (J Yan).
Author information
Authors and Affiliations
Contributions
J Yan designed the study, performed data management, and drafted the paper. J Yu and YJ L participated in the study design and performed the experiments and data analysis. W Z, X Y, and WQ T performed the experiments. HP L and SY L participated in the study. W G designed the MSC−/− mouse, provided several important suggestions, and improved English writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All animal procedures were conducted according to the guidelines and with the approval of the Army Medical University (Third Military Medical University) Laboratory Animal Management Committee and approved by the Ethics Committee.
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licen ses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Liu, Y., Zhang, W. et al. Musculin Deficiency Aggravates Colonic Injury and Inflammation in Mice with Inflammatory Bowel Disease. Inflammation 43, 1455–1463 (2020). https://doi.org/10.1007/s10753-020-01223-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01223-y